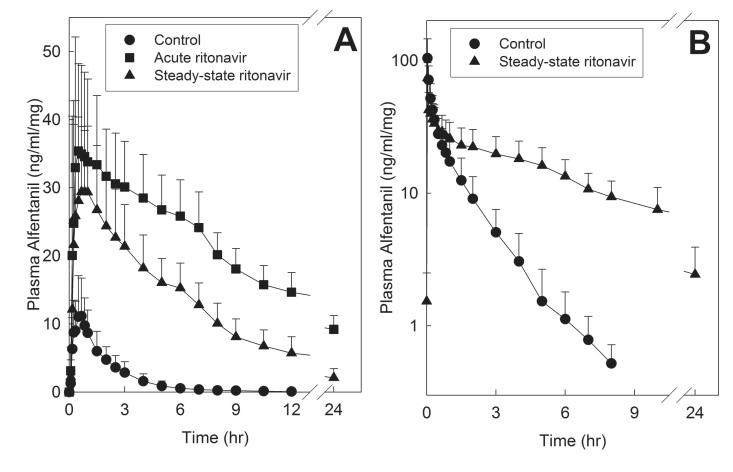
Figure 4.
Effect of acute and steady-state ritonavir on first-pass and hepatic CYP3A activity, assessed using alfentanil as a CYP3A probe. Shown are dose-adjusted alfentanil concentrations after (A) oral and (B) intravenous administration. Subjects received 43 and 4.3 μg/kg oral ALF at the control and ritonavir sessions, respectively, and 15 and 5 μg/kg IV alfentanil at the control and ritonavir sessions, respectively. Each data point is the mean ± SD (n=11).