Table 2.
Changes in hydration free energy on adding a chlorine, or moving chlorines, for selected pairs of ethane derivatives. Shown are calculated and experimental hydration free energy changes, as well as the portion of the calculated change due to electrostatics (ΔΔGcalc,e) and the portion due to changing Lennard-Jones interactions (ΔΔGcalc,LJ). All calculated values are from the MP2/SCRF set.
| Transformation | ΔΔGexpt | ΔΔGcalc | ΔΔGcalc,e | ΔΔGcalc,LJ | |
|---|---|---|---|---|---|
| (a) |
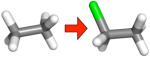
|
−2.25 ± 0.14 | −2.74 ± 0.01 | −2.31 ± 0.01 | −0.42 ± 0.01 |
| (b) |
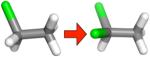
|
−0.49 ± 0.14 | −0.32 ± 0.01 | −0.08 ± 0.01 | −0.24 ± 0.01 |
| (C) |
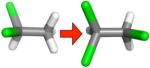
|
− 1.09 ± 0.14 | −0.34 ± 0.05 | −0.06 ± 0.01 | −0.29 ± 0.04 |
| (d) |
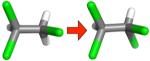
|
−0.40 ±0.14 | 0.21 ± 0.05 | 0.40 ± 0.01 | −0.20 ± 0.03 |
| (e) |

|
−0.92 ±0.14 | −1.00 ± 0.01 | −0.81 ± 0.01 | −0.19 ± 0.01 |
| (f) |
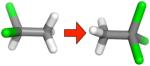
|
0.62 ±0.14 | 0.88 ± 0.01 | 1.0 ± 0.01 | −0.14 ± 0.01 |
| (g) |
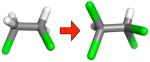
|
−0.57 ± 0.14 | 0.87 ± 0.02 | 1.16 ± 0.01 | −0.29 ± 0.02 |
