Fig. 2.
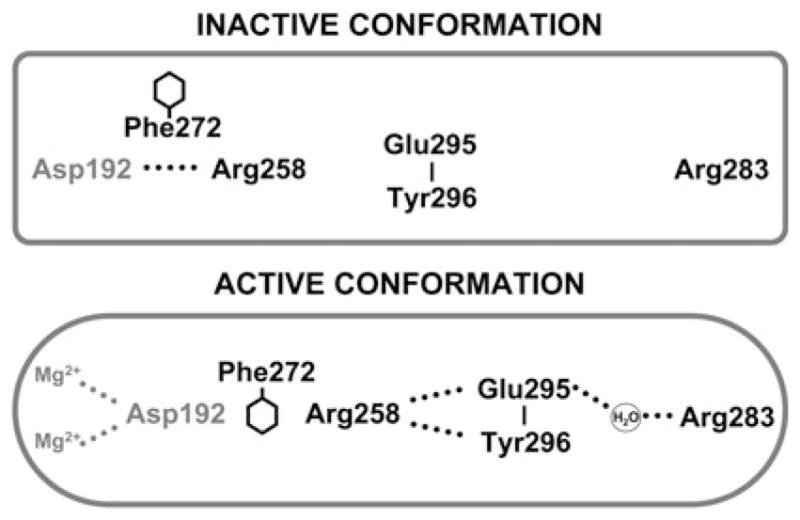
Altered side-chain interactions accompanying subdomain closure of pol β. In the open inactive conformation, Arg283 does not interact with other key residues, but in the closed active conformation, it interacts with the templating (coding) base, the upstream template nucleotide (not illustrated), and with Glu295 (indirectly). Thus, the position of the subdomain can be structurally transmitted to the active site through a series of interactions involving Arg283 permitting Asp192 to coordinate both active-site Mg2+ ions. This is also accompanied by altered interactions of Glu295/Tyr296 with Arg258 in the open (inactive) and closed (active) forms. Phe272 is postulated to transiently interfere with interactions between Asp192 and Arg258 permitting an interaction with Glu295/Tyr296. Residues of the subdomain that closes are indicated in boldface text. This figure was reproduced from Ref. [13] with permission
