Figure 6.
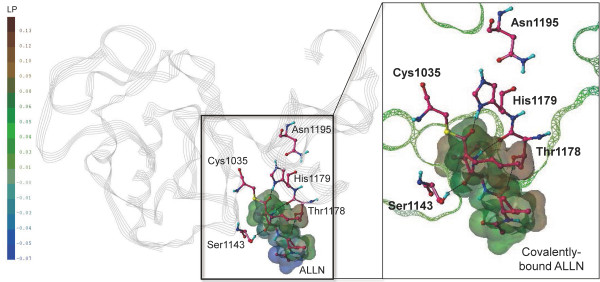
Refined model of Pf-calpain subdomain IIa covalently bound with ALLN. The secondary structure of Pf-calpain is displayed in grey line ribbon. The catalytic triad residues, Cys1035, His1179, and Asn1195, are displayed in ball-and-stick with the carbon color of hotpink. ALLN, covalently bound with Cys1035, was represented in ball-and-stick with the carbon color of hotpink, and its van der Waals surface was created by MOLCAD. In the enlarged view, the Connolly surface of Pf-calpain is displayed in mesh, and z-clipped from back and forth for the visual convenience. The molecular surfaces of the protein and ligand are colored by lipophilic potential (LP). Figure is generated by the PyMOL Molecular Graphics System v.1.3.
