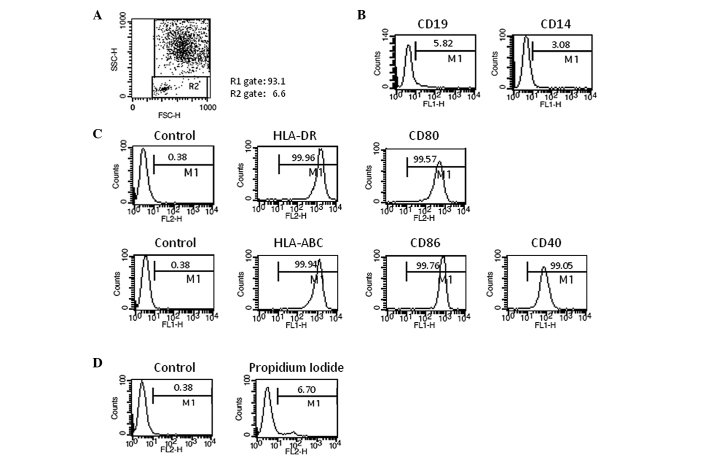
Figure 2.
Quality control of DC vaccine. DC vaccines generated in the institutional GMP facility were analyzed by FACS. (A) Cell size (FSC) and granularity (SSC) were analyzed in FACS light scattered plots. (B) Purity was assessed by measuring the contamination of CD14+ and CD19+ cells among DC gated cells. (C) The expression of MHC class I (HLA-ABC), MHC class II (HLA-DR), and costimulatory molecules such as CD80, CD86, and CD40 was assessed by FACS. The percentages of positive cells are indicated. (D) DC viability was assessed by propidium iodide exclusion methods.