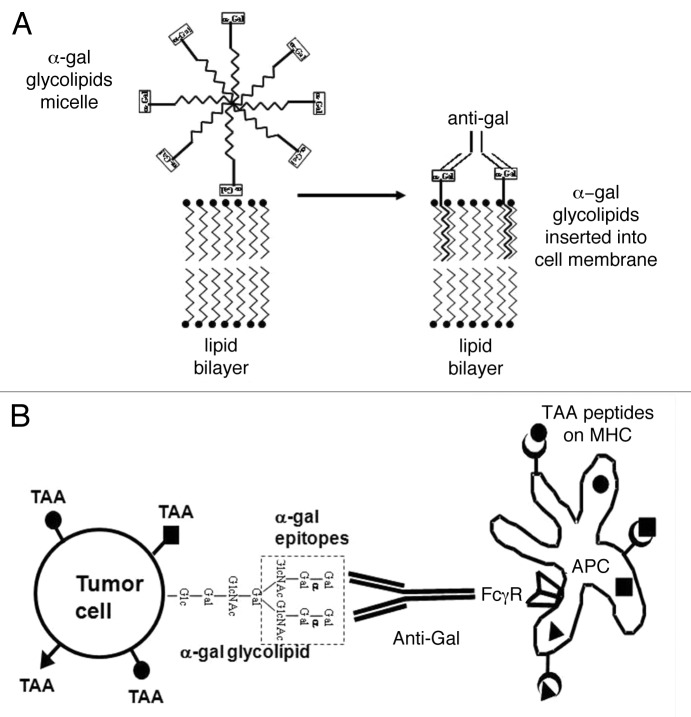
Figure 1. Conversion of tumors into vaccines by the intratumoral injection of α-gal glycolipids. (A) Insertion of α-gal glycolipids into cell membranes of injected tumors. α-gal glycolipids dissolved in the form of micelles (hydrophobic ceramide tails form the core of the micelle and hydrophilic carbohydrate chains protrude into the surrounding aqueous environment) are injected into tumors. These glycolipids spontaneously insert into the outer lipid layer of the plasma membrane. Multiple α-gal epitopes (rectangles) bind natural anti-Gal antibodies, which reach the injection site from ruptured capillaries. This interaction activates the complement system and generates chemotactic peptides that promote the migration of antigen-presenting cells (APCs) to the treated tumor. (B) Anti-Gal mediated targeting of tumor cells for uptake by antigen-presenting cells. APCs bind via their Fcγ receptors (FcγRs) to the Fc portion of anti-Gal antibodies coating tumor cells with inserted α-gal glycolipids. This interaction stimulates APCs to internalize intact or lysed tumor cells and their TAAs. APCs transport internalized TAAs to regional lymph nodes, process them and present the multiple autologous and potentially immunogenic TAA-derived peptides in association with MHC Class I and Class II molecules for the activation of TAA-specific T CD8+ and CD4+ cells, respectively. These activated T cells mediate a protective antitumor immune response.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.