Abstract
The ability of T cells to recognize a vast array of antigens enables them to destroy tumor cells while inflicting minimal collateral damage. Nevertheless, tumor antigens often are a form of self-antigen, and thus tumor immunity can be dampened by tolerance mechanisms that evolved to prevent autoimmunity. Since tolerance can be induced by steady-state antigen-presenting cells that provide insufficient co-stimulation, the exogenous administration of co-stimulatory agonists can favor the expansion and tumoricidal functions of tumor-specific T cells. Agonists of the co-stimulatory tumor necrosis factor receptor (TNFR) family members CD134 and CD137 exert antitumor activity in mice, and as monotherapies have exhibited encouraging results in clinical trials. This review focuses on how the dual administration of CD134 and CD137 agonists synergistically boosts T-cell priming and elaborates a multi-pronged antitumor immune response, as well as how such dual co-stimulation might be translated into effective anticancer therapies.
Keywords: CD134, CD137, CD4 T cell, dual costimulation, immunotherapy
Introduction
The ultimate goal of cancer research is to develop therapeutic strategies that specifically target tumor cells with minimal collateral damage to healthy tissue. The discovery of common mutations that drive tumorigenesis has enabled the development of drugs that produce dramatic clinical responses - at least in specific clinical settings - along with minimal side effects. Examples of this approach include the tyrosine kinase inhibitor imatinib (Gleevec®), which targets the chronic myelogenous leukemia (CML)-specific kinase BCR-ABL,1 and PLX4032, which inhibits mutated BRAF in a subset of metastatic melanoma patients.2 Despite these successes, non-specific approaches to cancer such as chemo- and radiotherapy, which impart substantial toxic side effects, remain the standard of care for the vast majority of cancer patients.
One of the hallmarks of cancer is genetic instability, resulting from chromosomal rearrangements and defects in DNA repair mechanisms that normally operate during DNA replication.3,4 Genetic mutations can give rise to new antigenic determinants that are selectively expressed by tumor, but not by healthy, cells, the number of which likely increases with disease progression.5 The random recombination of genes encoding T-cell receptor (TCR) chains endows the T-cell compartment of the adaptive immune system with a diverse repertoire of specificities. These can recognize short peptides derived from virtually any microbe when complexed with major histocompatibility (MHC) molecules expressed on the surface of infected cells or specialized antigen-presenting cells (APCs).6,7 This diversity also raises the potential for T cells to recognize tumor-specific peptides, which has fueled the development of therapeutic strategies to induce antitumor T-cell immunity.
Immunosuppressive nature of the tumor microenvironment
Although the immune system (and T cells in particular) can recognize tumor antigens, the high prevalence of cancer indicates that tumors must activate immunosuppressive mechanisms that thwart naturally arising antitumor T-cell responses. CD4+ T cells classically function as “helpers” to facilitate the function of cytotoxic CD8+ T cells (CTLs), natural killer (NK) cells, B cells and macrophages.8-11 It thus seemed paradoxical when North and colleagues found that depleting CD4+ T cells from tumor-bearing mice could promote the regression of advanced neoplastic lesions.12,13 It was subsequently found that these tumor-induced immunosuppressive T cells (termed regulatory T cells or Tregs) are defined by the constitutive expression of CD25, the α chain of the interleukin (IL)-2 receptor (which confers high affinity for IL-2),14 as well as the forkhead transcription factor FOXP3.15,16 CD25+FOXP3+ Tregs can suppress autoreactive T cells and control the magnitude of pathogen-specific T-cell responses.17,18 Tregs can be recruited into the tumor microenvironment, where their presence correlates with unfavorable prognosis, by factors such as the chemokine CCL22.19,20 Tregs appear to impede the immune control of tumor growth through multiple mechanisms. For instance, they can promote the expression of anti-inflammatory cytokines within the tumor microenvironment and suppress the ability of tumor-infiltrating CD8+ T cells to mediate antitumor effector functions.21,22
In addition to Tregs, there are several other immunosuppressive cell types that can localize within the tumor microenvironment. For instance, tumor infiltrating myeloid-derived suppressor cells (MDSCs) can desensitize tumor-specific CD8+ CTLs to cognate antigens by inducing covalent modifications of their TCR through the release of reactive oxygen species and peroxynitrite.23 In addition, tumor-associated macrophages (TAMs) can facilitate tumor angiogenesis, invasiveness and metastasis.24 Tregs, MDSCs, TAMs and other myeloid-derived cells function within an intricate, but not yet fully understood, immunosuppressive network within the tumor microenvironment.25
Tumor-reactive T cells and tolerance
To prevent autoimmunity, the bulk of developing T cells expressing self-reactive TCRs undergo negative selection in the thymus.26,27 A variety of peripheral tolerance mechanisms such as deletion,28 functional inactivation (i.e., anergy29) and suppression by Tregs17 control the activity of T cells recognizing self antigens that are not presented in the thymus. These peripheral tolerance mechanisms are not necessarily mutually exclusive, and in fact are likely to be deeply interrelated. For instance, self-reactive T cells often become anergic prior to undergoing deletion30,31 and under certain conditions anergic T cells express regulatory functions.32
Dendritic cells (DCs) are a central player in programming peripheral T-cell tolerance. Paradoxically, these bone marrow-derived APCs were originally defined by their potent ability to prime pathogen-specific T cells to develop effector and memory functions. Thus, in addition to their ability to efficiently acquire, process and present peptide epitopes from microbe-infected cells, DCs are induced by pathogen-associated molecular patterns (PAMPs) to express co-stimulatory ligands and cytokines that provide critical signals to enable antigen-stimulated naïve T cells to proliferate and develop effector functions.33-35 Co-stimulatory ligands principally belonging to the Ig superfamily such as CD80 (B7.1) and CD86 (B7.2) bind the co-stimulatory receptor CD28 on antigen-stimulated T cells to induce the initial phase of T-cell activation.36-38 DCs can also supply a second wave of co-stimulatory signals, mainly via members of the tumor necrosis factor receptor (TNFR) superfamily, which provide antigen-stimulated T cells with anti-apoptotic signals as well as with signals that program effector function and the capacity to form memory cells.39,40 Importantly, in steady-state conditions, DCs only express low levels of CD80 and CD86 and little-to-no levels of TNFR family members or cytokines such as IL-12. Thus, when steady-state DCs acquire and present self-antigens, their low expression of co-stimulatory ligands and cytokines causes cognate self-reactive T cells to undergo an abortive proliferative response that culminates in anergy, deletion or in the development of regulatory functions.41-44
Although the immune system can - at least theoretically - respond to tumor antigens, there is a fundamental difference between the targets of tumor-specific, as opposed to pathogen-specific, responses. Thus, pathogen-derived antigens are typically associated with PAMPs that potently activate DCs to prime cognate T cells, while tumor antigens are a form of self antigen, and thus are typically presented under steady-state conditions that are associated with the induction of tolerance. An exception to this rule are antigens that derive from oncogenic viruses such as the human papillomavirus45 and Epstein-Barr virus,46 which promote cervical tumors and B-cell lymphomas, respectively. Many tumor-associated antigens are non-mutated self antigens that are expressed on both tumors as well as healthy cells. Prototypical examples of such tumor differentiation antigens are the melanocytic antigens tyrosinase,47,48 TRP-249,50 and Pmel-17/gp100.51,52 Vaccination strategies against these antigens can elicit CD8+ CTLs with the potential to destroy both melanoma cells as well as healthy melanocytes (i.e., resulting in autoimmune vitiligo).53,54 Nevertheless, the quality and magnitude of CTL responses to tumor differentiation antigens can be limited by peripheral tolerance, as induced by the same antigens derived from healthy tissue.55-57 Taken together, T-cell tolerance mechanisms can limit the magnitude and effectiveness of antitumor immunity directed toward tumor differentiation antigens. In this scenario, when tolerance is overcome, autoimmunity may be a side effect of tumor immunity.
Tumor differentiation antigens most often induce T-cell tolerance prior to the onset of tumorigenesis, because they are expressed on healthy tissues.58 Thus, it might seem reasonable that T cells would be less tolerant of tumor-specific antigens that derive from mutated self proteins that are not encoded in the genome of healthy cells. Nevertheless, peripheral T-cell tolerance to tumor-specific antigens occurs in both transplantable and autochthonous tumor models.59-62 Specifically, steady-state DCs can acquire tumor antigens and present them in the same tolerogenic manner as self antigens deriving from healthy tissues.63,64 This said, tolerance does not always occur,65 and in some situations cognate T cells can develop tumoricidal effector functions.66-68 The ability of tumors to prime rather than tolerize cognate T cells may depend on whether they release inflammation-inducing endogenous danger-associated molecular patterns (DAMPs) such as heat shock proteins,69 uric acid,70 or HMGB171 when they metastasize or invade across basement membranes.72 Tumors that prime cognate T cells typically engage immunosuppressive mechanisms to dampen the activity of infiltrating tumor-specific effector T cells (see above). Additionally, tumors can undergo “immunoediting,” a process whereby some cells within a heterogeneous tumor mass are eliminated by effector T cells and innate immune cells while another cell population that has downregulated cognate T-cell epitopes and ligands for innate immune cells expands to form a non-immunogenic tumor.73 Notably, tumor-specific T cells can mediate immunoediting while simultaneously undergoing tolerance,74 which may help to explain the early paradoxical observation that human cancer patients often harbor clonally expanded populations of anergic tumor-specific T cells.75 Hence, the initial phase of the antitumor immune response appears to promote the outgrowth of non-immunogenic tumors.
Co-stimulatory agonists program T cells encountering non-immunogenic antigens to expand and develop tumoricidal effector functions
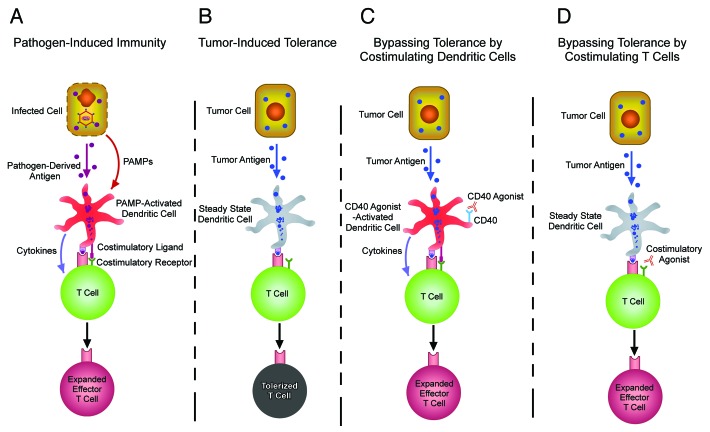
Because tumor-reactive T cells can undergo tolerization when tumors elicit insufficient inflammation to induce co-stimulatory activity on DCs (Fig. 1A,B), agonistic monoclonal antibodies to co-stimulatory ligands and receptors have been used to program tumor-reactive T cells to expand and acquire tumoricidal effector functions (Fig. 1C,D). For instance, CD40 is a TNFR family member expressed on DCs that - when bound by the its ligand (CD40L or CD154) expressed on activated CD4+ helper T cells - upregulates MHC molecules, co-stimulatory ligands such as CD80 and CD86, as well as cytokines such as IL-12, and hence has the potential to effectively prime CD8+ CTLs.76-79 CD40 agonist thus can compensate for the absence of activated CD4+ helper T cells, and - similar to classical adjuvants (e.g., PAMPs that bind Toll-like receptors) - can induce DCs to express co-stimulatory ligands and release cytokines enabling effective T-cell priming (Fig. 1C).80-82 A reciprocal approach is to employ agonists to co-stimulatory receptors expressed on T cells (Fig. 1D). An example is the TNFR family member CD134 (OX40), which is expressed on T cells following TCR stimulation83,84 and normally bound by CD252 (OX40L) on activated, but not steady-state, DCs.85 When naïve T cells are primed by cognate peptide-presenting activated DCs, the CD252-CD134 interaction initiates signals that program T-cell survival and effector differentiation.84,86-88 CD134 agonists thus enable T cells stimulated by steady-state DCs that lack CD252 to avoid tolerance and undergo expansion and effector differentiation.89-94 Importantly, agonists to several TNFR co-stimulatory family members such as CD40,81,82 CD134,93,95 CD137,96 CD2797 and GITR98 can elicit antitumor immunity in mice. Further, humanized agonists to CD134 and CD137 are being tested in clinical trials to treat human neoplasms.99,100
Figure 1. Co-stimulatory agonists enable T cells responding to tolerogenic tumor antigens to undergo expansion and effector differentiation. (A) Pathogen-associated molecular patterns (PAMPs) activate dendritic cells (DCs) presenting microbial peptides to express cytokines and co-stimulatory ligands that program T-cell expansion and effector differentiation. (B) In steady-state conditions, DCs acquire and present tumor antigens, but the absence of PAMPs results in the sub-optimal expression of cytokines and co-stimulatory ligands, causing cognate tumor-specific T cells to undergo tolerization. (C) CD40 agonists can activate DCs presenting tumor antigens to express cytokines and co-stimulatory ligands that program T-cell expansion and effector differentiation. (D) Agonists to co-stimulatory receptors program T cells responding to tumor antigens presented by steady-state DCs to undergo expansion and effector differentiation. A potential advantage of the approach described in (D), as compared with that depicted in (C), is that potentially toxic cytokines produced by DCs such as IL-12 are likely to be elaborated in much lower amounts.
Co-stimulatory agonists can be applied in synergistic therapeutic combinations
The TNFR superfamily contains at least 29 receptors (and 19 ligands), several pairs of which play a role in T-cell co-stimulation: CD134-CD252, CD27-CD70, CD30-CD30L, CD137-4-1BBL, LIGHT-HVEM, CD40-CD40L and GITR-GITRL.39,40,101 This plethora of co-stimulatory mechanisms likely reflects the fact that individual pathways program unique facets of T-cell functions. For instance, although CD134 and CD137 are both expressed on activated CD4+ and CD8+ T cells, CD134 co-stimulation generally has a greater impact on CD4+ T-cell function84,102-104 while CD137 more significantly impacts CD8+ T cells.104-109 Further, innate immune cell types such as DCs and NK cells are more responsive to CD137 than CD134.110-114 The particular combination of co-stimulatory signals engaged during an immune response is therefore likely to influence the overall functional outcome of T-cell priming. Given that the most effective antitumor immune responses may involve the recruitment of multiple immune effector arms, the administration of co-stimulatory agonists in combination may elicit the most potent therapeutic responses.
The potential to elicit synergistic effects by multiple co-stimulatory pathways may depend (at least in part) on the ability of each pathway to trigger unique downstream signaling events. Thus, although all TNFR family members initiate cytoplasmic signaling through one or more of the six TNFR-associated factors (TRAF1-6) that interact with their intracellular domains and engage several downstream signal transduction pathways such as ERK-, JNK-, p38- and NFκB-dependent pathways, the distinct TNFR family members engage different combinations of TRAFs.40,100,101,115 Furthermore, TNFR family members differ in their expression patterns. For instance, CD40 is principally expressed on APCs,116-118 CD134 is transiently expressed on TCR-stimulated conventional T cells83,84 but constitutively present on the surface of FOXP3+CD25+ Tregs,119 while CD137 can be expressed on T cells, NK cells, DCs and other innate cells.110,111,113 Thus, the potential for individual TNFR agonists to exert distinct effects in shaping T-cell responsiveness may be determined by a combined effect of triggering unique combinations of intracellular signaling pathways in distinct cell subsets.
One example of an effective combination therapy involves the co-administration of CD40, CD137 and DR5 (apoptosis-inducing receptor for TNF-related apoptosis-inducing ligand, TRAIL) agonists, eliciting a CD8+ T cell-dependent eradication of pre-established tumors in mice.120 In analyzing the potential of various combinations of CD40, CD134 and CD137 agonists to program CD8+ T cell responsiveness, multiple mouse studies have revealed that the combination of CD134 plus CD137 agonists was particularly effective in boosting CD8+ T-cell expansion, effector function and antitumor immunity.121-124 The fact that the effects of CD134 plus CD137 co-stimulation were not simply additive was demonstrated both by their synergistic effect in boosting CD8+ T-cell clonal expansion as well as by the necessity for CD137 co-stimulation to enable CD134 agonist to program CD8+ T cells to differentiate into interferon γ (IFNγ) superproducers.125
CD134 plus CD137 co-stimulation programs a multi-pronged antitumor immune response
A major advantage of CD134 plus CD137 dual co-stimulation therapy is its potential to elicit a multi-pronged antitumor response. A first prong of this program is a robust CD8+ CTL response.121,123-125 A second one stems from ability of CD137 alone to activate innate immune cells such as DCs and NK cells.110-114
A third, and unexpected, prong is the ability of dual co-stimulation to program CD4+ T cells to differentiate into cytotoxic TH1 effectors that not only produce IFNγ, but also kill target cells presenting cognate MHC class II-restricted peptides.126 Cytotoxic functions are classically associated with CD8+ CTLs and NK cells,127,128 and although it was known that cultured CD4+ T cells can develop cytotoxic potential in vitro,129,130 it has only recently become clear that these cells can be induced in vivo in response to certain infections.131-133 Cytotoxic CD4+ T cells might be useful in targeting MHC class II+ tumors and notably melanomas, which can express MHC class II molecules134 but have a propensity to downregulate their class I counterparts.135 Indeed, cytotoxic CD4+ TH1 cells can effectively target murine melanoma.136,137 Moreover, CD134 plus CD137 co-stimulated CD4+ T cells exert antitumor activity against murine melanoma.126
Given that humanized CD134 and CD137 agonists are being tested in human cancer patients,99,100 it will be important to understand how CD134 plus CD137 co-stimulation induces cytotoxic CD4+ TH1 cells and fully explore their therapeutic potential. Consistent with the notion that CD4+ T cells are typically more responsive to CD134 co-stimulation,84,102-104 CD134 agonist, but not 4-1BB, is sufficient to program the cytotoxic CD4+ TH1 functional profile (i.e., the ability to express IFNγ and the apoptosis-inducing serine protease granzyme B, GzmB).126 Nevertheless, the addition of CD137 co-stimulation maximizes the clonal expansion of cytotoxic CD4+ TH1 cells,126 an effect that might promote their therapeutic potential. Mechanistically, cytotoxic TH1 differentiation depends upon the cytokine IL-2 and the T-box transcription factor Eomesodermin (Eomes).126 Eomes was initially characterized as a CD8+ T cell-specific factor that drives the expression of GzmB, perforin and IFNγ,138,139 indicating that CD134 plus CD137 co-stimulation programs a sort of “CD8-like” CD4+ T cells by inducing a transcription factor normally expressed by CD8+ T cells in a restricted fashion. An intriguing facet of this dual co-stimulation response is that while antigen-responding CD4+ T cells undergo cytotoxic TH1 differentiation, antigen-non-responding (bystander) T cells are also induced to express GzmB.126 A common reason for the failure of T cell-based antitumor therapies is the outgrowth of antigen-loss variant tumor cells that lack expression of the targeted epitopes.74,140-144 Given that dual co-stimulation-programmed GzmB+ bystander T cells have a diverse polyclonal TCR repertoire, they may have the potential to target such antigen-loss variant tumor cells.
A fourth prong derives from the fact that CD134 plus CD137 co-stimulation can program effector T cells to elaborate TCR-independent effector functions. Recently, it has been shown that dual-co-stimulated CD8+ T cells produce prodigious amounts of IFNγ when exposed to IL-33 in the context of IL-12.145 Unlike IL-12, active IL-33 is typically released by necrotic cells to alert the immune system of danger.146,147 This new finding has yet to be exploited in tumor models. Thus, the potential of IL-33 plus IL-12 administered directly into tumors to trigger dual-co-stimulated CD8+ effector T cells to secrete IFNγ may bypass the consistent problem of MHC downregulation by malignant cells,135 which theoretically precludes the TCR-triggered elaboration of effector functions. This concept provides a novel approach that may avoid toxic side effects associated with systemic high dose IL-12.148 Thus, dual co-stimulation may lower the overall threshold for effector cell activation by programming both TCR-dependent and -independent effector functions.
Potential therapeutic advantages and disadvantages of dual co-stimulation therapy
Like any experimental therapy, CD134 plus CD137 co-stimulation has both potential advantages and disadvantages. As described above, the strong therapeutic potential of this approach stems from a multi-pronged immune response that involves cells from the innate immune system, antigen-specific CD8+ CTLs, cytotoxic TH1 CD4+ cells, and bystander CTLs. This broad attack that engages both innate and adaptive immune components should help to minimize outgrowth of tumors resistant to individual (and even multiple) immune effector arms.
A common toxicity associated with T cell-based cancer therapies that target tumor differentiation antigens as autoimmune reactions directed against the healthy tissues from which the tumors develop.53,54,149,150 Notably, an antagonist to the T-cell immune checkpoint protein CTLA-4 (ipilimumab) that has recently received FDA approval for the treatment of advanced melanoma patients can elicit autoimmune side effects.151-155 Co-stimulatory modulators can also elicit antigen-unrelated immune toxicities. Thus, in a clinical trial testing the tolerability of a CD28 superagonist (that was expected to only activate Tregs) six out of six healthy volunteers experienced multiorgan failure in association with a storm of pro-inflammatory cytokines.156
Thus far, in a Phase I clinical trial, CD134 agonist monotherapy has not produced any toxicities99 while in Phase II trials CD137 monotherapy has exhibited both clinical efficacy as well as some degrees of liver toxicity.157 It has previously been shown that the careful titration of CD134 and CD137 agonists in mice significantly lowers the effective dose required to achieve optimal CD8+ T-cell responses.125 This approach may thus preserve beneficial antitumor activity while limiting adverse side effects. The use of lower doses of agonistic antibodies might also limit the development of human anti-chimeric antibodies (HACA),158 hence allowing for multiple dosing. This strategy seems less feasible with single co-stimulators,159 and thus represents a potential in-built clinical advantage of dual co-stimulation-based therapeutic approaches over monotherapies.
Translating dual co-stimulation into effective anticancer therapies
The successful translation of CD134 plus CD137 co-stimulation into an effective therapy for human cancer will be facilitated by efforts in several areas. First, an increased understanding of how such agonists regulate human immune responses is required. The bulk of our understanding of how these co-stimulatory pathways stimulate immunity derive from mouse models. Although it is known that CD134 and CD137 can co-stimulate human T cells160,161 and results from clinical trials so far support the antineoplastic potential of individual humanized agonists,99,100 it will be critical to gain deeper insight into how dual co-stimulation impacts human immune responses. Preliminary studies demonstrating that CD134 plus CD137 co-stimulation boosts the priming of human T cells in vitro beyond the effects of either co-stimulator alone162 support a potential of dual co-stimulation therapy to elicit therapeutic responses in cancer patients.
It will also be important to further dissect how CD134 and CD137 mechanistically synergize to program robust effector T-cell responses. For instance, CD137 co-stimulation (occurring on either CD8+ T cells or innate immune cells) enables CD134 agonist to elicit supereffector CD8+ T cells that express high levels of both IFNγ and TNF.125 However, it is not clear how CD137 engages the CD134 pathway. In addition, CD134, but not CD137, induces robust IFNγ and GzmB expression in CD4+ T cells, while the addition of CD137 co-stimulation maximizes the clonal expansion of cytotoxic CD4+ TH1 cells.126 Given the overlap in the intracellular signaling pathways initiated by the these two TNFR family members (both involving TRAFs), it is surprising that they play distinct (rather than simply additive) roles in programming both CD4+ and CD8+ T-cell responses. One possibility is that subtle differences in the respective downstream signaling pathways confer distinct effects in programming T-cell responsiveness. Also, critical signaling events might occur on different cells. Specifically, the mostly T cell-restricted expression pattern of CD13483,84 suggest that CD134 co-stimulation presumably needs to occur on antigen-stimulated T cells. In contrast, numerous innate cell types express CD137110,111,113 and CD137 co-stimulation in innate cells can impact specific T-cell responses.125 Thus, the synergistic effect of CD134 and CD137 co-stimulation may occur through both cell-extrinsic and cell-intrinsic mechanisms.
Advanced cancer is inherently difficult to treat in part due to its high degree of genetic instability. This can lead to the outgrowth of tumor clones that are resistant not only to a particular immunotherapy, for instance owing to variants that have downregulated MHC class I molecules or specific CTL epitopes,74,140-144 but also to non-immunological therapies such as oncoprotein-targeted small molecules like imatinib163 and BRAF inhibitor.164-166 In some cases, it has been possible to control chemoresistant tumors using drug combinations that differentially target the same oncogenic pathway (e.g., using dasatinib to treat imatinib-resistant tumors163). As discussed above, dual co-stimulation therapy may be effective in limiting the outgrowth of antigen-loss variants, given its potential to engage multiple tumoricidal immune effector arms. Nevertheless, dual co-stimulation might become more effective if combined with antagonists to immune checkpoint molecules such as CTLA-4 and PD-1, which themselves possess therapeutic potential151-155,167 but elicit more potent therapeutic responses when combined with immune stimulators.168-171 Another potential area to exploit is the use of alarmins (e.g., IL-33) or cytokines that dual-co-stimulated effector T cells have become able to respond to.145
Combining dual co-stimulation with standard therapies might produce the most beneficial clinical responses, as the genetic alterations conferring resistance to these different treatment modalities are very unlikely to overlap. Support for this idea come from studies in which tumor vaccines elicited more durable therapeutic responses against lymphoma when given following bone marrow transplantation,172 and against solid tumors when given following the administration of chemotherapeutic drugs.173,174 Importantly, besides establishing states of minimal residual disease (and hence minimize the tumor burden for the immune response to eliminate), these standard of care modalities may also promote antitumor immune responses.172,174 Anti-lymphoma vaccines administered following bone marrow transplantation may elicit stronger antitumor T-cell responses due to lymphopenia resulting from the pre-conditioning regimen.175,176 Chemotherapeutic drugs may augment antitumor immunity by eliciting the production of type I interferons177 or by inducing the release of DAMPs from dying tumor cells, which facilitate tumor antigen uptake by DCs and their activation.71,178,179 The fact that a CD134 agonist and the chemotherapeutic drug cyclophosphamide synergize in controlling melanoma growth by inducing Treg apoptosis within tumors as well as by priming tumoricidal CD4+ effector T cells180,181 suggests that it may be worthwhile to examine the therapeutic efficacy of combining CD134 plus CD137 co-stimulation with chemotherapy.
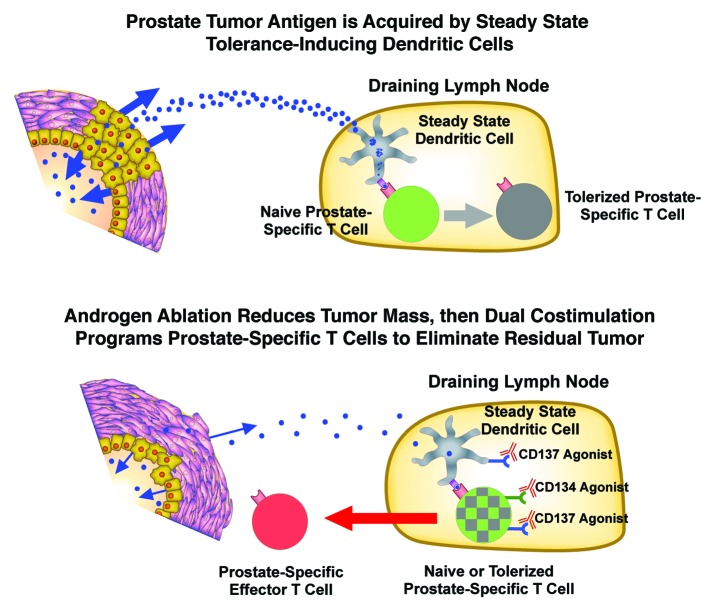
Prostate cancer, the most common malignancy in American men,182 is an attractive target for T cell-based therapies in part because potential autoimmune side effects directed against healthy prostatic tissue should be tolerable, given the non-vital nature of the prostate gland. Further, T cell-based therapies might be particularly effective when given in conjunction with the standard of care treatment for advanced prostate cancer (Fig. 2). Thus androgen ablation/deprivation is used for advanced disease, which cannot be treated by surgery or radiation.183 Although androgen ablation is typically effective in initially reducing tumor burden, because most prostate tumor cells depend upon androgens to grow and survive, disease inevitably recurs due to the outgrowth of tumor clones in which the androgen receptor signaling axis functions even in the absence of normal androgen levels or in the presence of anti-androgens.184,185 The development of autochthonous prostate tumors in mice induces T-cell tolerance to prostate-specific antigens, but - importantly - androgen ablation diminishes this effect, apparently by reducing tumor mass and hence the quantity of tumor antigen available for presentation by steady-state tolerogenic DCs.64,186 Clinical trials are currently testing the idea that T cell-based prostate cancer therapies may be most effective when administered soon after androgen ablation.187 Androgen ablation may improve the clinical outcome of immune therapy (at least in part) by reducing the number of tumor cells to be eliminated by the immune system. Additionally, androgen ablation reverses age-related thymic atrophy,188 and is therefore likely to increase the number of newly generated naïve prostate-specific T cells available for priming. Furthermore, CD134 agonists can reverse pre-existing T-cell anergy,189,190 and thus dual co-stimulation may also be able to engage previously tolerized prostate-specific T cells.
Figure 2. Dual co-stimulation administered following androgen ablation may be an effective combination therapy to treat prostate cancer. Top, prostate tumor antigens presented by steady-state dendritic cells (DCs) in the draining lymph node program prostate-specific T cells to undergo tolerization. Bottom, androgen ablation induces a state of minimal residual disease by causing the majority of tumor cells to undergo cell death. Dual co-stimulation therapy may program prostate-specific T cells to expand, acquire effector functions, and eliminate the residual tumor. Since androgen ablation also induces the regeneration of the aged thymus, these prostate-specific T cells may be naïve recent thymic emigrants (green). Dual co-stimulation may also engage previously tolerized prostate-specific T cells (gray) since CD134 agonists can reverse pre-existing T cell anergy.
Concluding Remarks
As discussed above, translating dual co-stimulation into an effective anticancer therapy will require the resolution of several outstanding questions: (1) how CD134 and CD137 synergize at the genetic and biochemical level to program TCR-dependent effector functions beyond those elicited by single co-stimulators; (2) whether dual co-stimulation-programmed TCR-independent effector functions (e.g., the release of IL-33) can be exploited for therapeutic use; and (3) whether potential synergies between dual co-stimulation and other immune modulators (e.g., CTLA-4 antagonist) as well as non-immune-based therapeutic modalities may results in superior antineoplastic effects.
It is commonly known that “two heads are better than one” and we propose that dual co-stimulation is better than mono co-stimulation. The capacity to administer lower doses of agonists while achieving greater clinical benefit is a therapeutic goal that can be achieved by triggering specific combinations of co-stimulatory pathways. As not all co-stimulatory pathways fit into this category, a goal for the field is to uncover why some agonistic combinations work better than others. Understanding the basis of this synergism will allow for the rationale design of small molecules that operate similarly to humanized reagents and potentially spawn a more personalized approach to cancer treatment. In sum, many co-stimulatory pathways are known, but finding the right combination of co-stimulation or cytokines for selected clinical circumstances may be the holy grail for the efficient treatment against different forms of cancer that strike humans.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
The research activity in the authors’ laboratories is supported by NIH grants RO1CA109339 and RO1AI094640.
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/22837
References
- 1.Druker BJ, Guilhot F, O’Brien SG, Gathmann I, Kantarjian H, Gattermann N, et al. IRIS Investigators Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N Engl J Med. 2006;355:2408–17. doi: 10.1056/NEJMoa062867. [DOI] [PubMed] [Google Scholar]
- 2.Flaherty KT, Puzanov I, Kim KB, Ribas A, McArthur GA, Sosman JA, et al. Inhibition of mutated, activated BRAF in metastatic melanoma. N Engl J Med. 2010;363:809–19. doi: 10.1056/NEJMoa1002011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lengauer C, Kinzler KW, Vogelstein B. Genetic instabilities in human cancers. Nature. 1998;396:643–9. doi: 10.1038/25292. [DOI] [PubMed] [Google Scholar]
- 4.Stephens PJ, Greenman CD, Fu B, Yang F, Bignell GR, Mudie LJ, et al. Massive genomic rearrangement acquired in a single catastrophic event during cancer development. Cell. 2011;144:27–40. doi: 10.1016/j.cell.2010.11.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Srivastava N, Srivastava PK. Modeling the repertoire of true tumor-specific MHC I epitopes in a human tumor. PLoS One. 2009;4:e6094. doi: 10.1371/journal.pone.0006094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schatz DG, Oettinger MA, Schlissel MS. V(D)J recombination: molecular biology and regulation. Annu Rev Immunol. 1992;10:359–83. doi: 10.1146/annurev.iy.10.040192.002043. [DOI] [PubMed] [Google Scholar]
- 7.Nikolich-Žugich J, Slifka MK, Messaoudi I. The many important facets of T-cell repertoire diversity. Nat Rev Immunol. 2004;4:123–32. doi: 10.1038/nri1292. [DOI] [PubMed] [Google Scholar]
- 8.Bevan MJ. Helping the CD8(+) T-cell response. Nat Rev Immunol. 2004;4:595–602. doi: 10.1038/nri1413. [DOI] [PubMed] [Google Scholar]
- 9.King C, Tangye SG, Mackay CR. T follicular helper (TFH) cells in normal and dysregulated immune responses. Annu Rev Immunol. 2008;26:741–66. doi: 10.1146/annurev.immunol.26.021607.090344. [DOI] [PubMed] [Google Scholar]
- 10.Khader SA, Gaffen SL, Kolls JK. Th17 cells at the crossroads of innate and adaptive immunity against infectious diseases at the mucosa. Mucosal Immunol. 2009;2:403–11. doi: 10.1038/mi.2009.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhu J, Yamane H, Paul WE. Differentiation of effector CD4 T cell populations (*) Annu Rev Immunol. 2010;28:445–89. doi: 10.1146/annurev-immunol-030409-101212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.North RJ. Cyclophosphamide-facilitated adoptive immunotherapy of an established tumor depends on elimination of tumor-induced suppressor T cells. J Exp Med. 1982;155:1063–74. doi: 10.1084/jem.155.4.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.North RJ, Awwad M. Elimination of cycling CD4+ suppressor T cells with an anti-mitotic drug releases non-cycling CD8+ T cells to cause regression of an advanced lymphoma. Immunology. 1990;71:90–5. [PMC free article] [PubMed] [Google Scholar]
- 14.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–64. [PubMed] [Google Scholar]
- 15.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–61. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 16.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–6. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 17.Sakaguchi S. Regulatory T cells: key controllers of immunologic self-tolerance. Cell. 2000;101:455–8. doi: 10.1016/S0092-8674(00)80856-9. [DOI] [PubMed] [Google Scholar]
- 18.Shevach EM. Certified professionals: CD4(+)CD25(+) suppressor T cells. J Exp Med. 2001;193:F41–6. doi: 10.1084/jem.193.11.F41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–9. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 20.Sato E, Olson SH, Ahn J, Bundy B, Nishikawa H, Qian F, et al. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc Natl Acad Sci U S A. 2005;102:18538–43. doi: 10.1073/pnas.0509182102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yu P, Lee Y, Liu W, Krausz T, Chong A, Schreiber H, et al. Intratumor depletion of CD4+ cells unmasks tumor immunogenicity leading to the rejection of late-stage tumors. J Exp Med. 2005;201:779–91. doi: 10.1084/jem.20041684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cao X, Cai SF, Fehniger TA, Song J, Collins LI, Piwnica-Worms DR, et al. Granzyme B and perforin are important for regulatory T cell-mediated suppression of tumor clearance. Immunity. 2007;27:635–46. doi: 10.1016/j.immuni.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 23.Nagaraj S, Gupta K, Pisarev V, Kinarsky L, Sherman S, Kang L, et al. Altered recognition of antigen is a mechanism of CD8+ T cell tolerance in cancer. Nat Med. 2007;13:828–35. doi: 10.1038/nm1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mantovani A, Sica A. Macrophages, innate immunity and cancer: balance, tolerance, and diversity. Curr Opin Immunol. 2010;22:231–7. doi: 10.1016/j.coi.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 25.Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol. 2012;12:253–68. doi: 10.1038/nri3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kappler JW, Roehm N, Marrack P. T cell tolerance by clonal elimination in the thymus. Cell. 1987;49:273–80. doi: 10.1016/0092-8674(87)90568-X. [DOI] [PubMed] [Google Scholar]
- 27.Hogquist KA, Baldwin TA, Jameson SC. Central tolerance: learning self-control in the thymus. Nat Rev Immunol. 2005;5:772–82. doi: 10.1038/nri1707. [DOI] [PubMed] [Google Scholar]
- 28.Krueger A, Fas SC, Baumann S, Krammer PH. The role of CD95 in the regulation of peripheral T-cell apoptosis. Immunol Rev. 2003;193:58–69. doi: 10.1034/j.1600-065X.2003.00047.x. [DOI] [PubMed] [Google Scholar]
- 29.Schwartz RH. T cell anergy. Annu Rev Immunol. 2003;21:305–34. doi: 10.1146/annurev.immunol.21.120601.141110. [DOI] [PubMed] [Google Scholar]
- 30.Webb S, Morris C, Sprent J. Extrathymic tolerance of mature T cells: clonal elimination as a consequence of immunity. Cell. 1990;63:1249–56. doi: 10.1016/0092-8674(90)90420-J. [DOI] [PubMed] [Google Scholar]
- 31.Rocha B, von Boehmer H. Peripheral selection of the T cell repertoire. Science. 1991;251:1225–8. doi: 10.1126/science.1900951. [DOI] [PubMed] [Google Scholar]
- 32.Jooss K, Gjata B, Danos O, von Boehmer H, Sarukhan A. Regulatory function of in vivo anergized CD4(+) T cells. Proc Natl Acad Sci U S A. 2001;98:8738–43. doi: 10.1073/pnas.151088898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–52. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 34.Jenkins MK, Khoruts A, Ingulli E, Mueller DL, McSorley SJ, Reinhardt RL, et al. In vivo activation of antigen-specific CD4 T cells. Annu Rev Immunol. 2001;19:23–45. doi: 10.1146/annurev.immunol.19.1.23. [DOI] [PubMed] [Google Scholar]
- 35.Janeway CA, Jr., Medzhitov R. Innate immune recognition. Annu Rev Immunol. 2002;20:197–216. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- 36.Jenkins MK, Taylor PS, Norton SD, Urdahl KB. CD28 delivers a costimulatory signal involved in antigen-specific IL-2 production by human T cells. J Immunol. 1991;147:2461–6. [PubMed] [Google Scholar]
- 37.Harding FA, McArthur JG, Gross JA, Raulet DH, Allison JP. CD28-mediated signalling co-stimulates murine T cells and prevents induction of anergy in T-cell clones. Nature. 1992;356:607–9. doi: 10.1038/356607a0. [DOI] [PubMed] [Google Scholar]
- 38.Norton SD, Zuckerman L, Urdahl KB, Shefner R, Miller J, Jenkins MK. The CD28 ligand, B7, enhances IL-2 production by providing a costimulatory signal to T cells. J Immunol. 1992;149:1556–61. [PubMed] [Google Scholar]
- 39.Croft M. Co-stimulatory members of the TNFR family: keys to effective T-cell immunity? Nat Rev Immunol. 2003;3:609–20. doi: 10.1038/nri1148. [DOI] [PubMed] [Google Scholar]
- 40.Watts TH. TNF/TNFR family members in costimulation of T cell responses. Annu Rev Immunol. 2005;23:23–68. doi: 10.1146/annurev.immunol.23.021704.115839. [DOI] [PubMed] [Google Scholar]
- 41.Hawiger D, Inaba K, Dorsett Y, Guo M, Mahnke K, Rivera M, et al. Dendritic cells induce peripheral T cell unresponsiveness under steady state conditions in vivo. J Exp Med. 2001;194:769–79. doi: 10.1084/jem.194.6.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kurts C, Cannarile M, Klebba I, Brocker T. Dendritic cells are sufficient to cross-present self-antigens to CD8 T cells in vivo. J Immunol. 2001;166:1439–42. doi: 10.4049/jimmunol.166.3.1439. [DOI] [PubMed] [Google Scholar]
- 43.Hagymasi AT, Slaiby AM, Mihalyo MA, Qui HZ, Zammit DJ, Lefrancois L, et al. Steady state dendritic cells present parenchymal self-antigen and contribute to, but are not essential for, tolerization of naive and Th1 effector CD4 cells. J Immunol. 2007;179:1524–31. doi: 10.4049/jimmunol.179.3.1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Luckashenak N, Schroeder S, Endt K, Schmidt D, Mahnke K, Bachmann MF, et al. Constitutive crosspresentation of tissue antigens by dendritic cells controls CD8+ T cell tolerance in vivo. Immunity. 2008;28:521–32. doi: 10.1016/j.immuni.2008.02.018. [DOI] [PubMed] [Google Scholar]
- 45.Lin KY, Guarnieri FG, Staveley-O’Carroll KF, Levitsky HI, August JT, Pardoll DM, et al. Treatment of established tumors with a novel vaccine that enhances major histocompatibility class II presentation of tumor antigen. Cancer Res. 1996;56:21–6. [PubMed] [Google Scholar]
- 46.Heslop HE, Rooney CM. Adoptive cellular immunotherapy for EBV lymphoproliferative disease. Immunol Rev. 1997;157:217–22. doi: 10.1111/j.1600-065X.1997.tb00984.x. [DOI] [PubMed] [Google Scholar]
- 47.Brichard V, Van Pel A, Wölfel T, Wölfel C, De Plaen E, Lethé B, et al. The tyrosinase gene codes for an antigen recognized by autologous cytolytic T lymphocytes on HLA-A2 melanomas. J Exp Med. 1993;178:489–95. doi: 10.1084/jem.178.2.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kang X, Kawakami Y, el-Gamil M, Wang R, Sakaguchi K, Yannelli JR, et al. Identification of a tyrosinase epitope recognized by HLA-A24-restricted, tumor-infiltrating lymphocytes. J Immunol. 1995;155:1343–8. [PubMed] [Google Scholar]
- 49.Wang RF, Appella E, Kawakami Y, Kang X, Rosenberg SA. Identification of TRP-2 as a human tumor antigen recognized by cytotoxic T lymphocytes. J Exp Med. 1996;184:2207–16. doi: 10.1084/jem.184.6.2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bloom MB, Perry-Lalley D, Robbins PF, Li Y, el-Gamil M, Rosenberg SA, et al. Identification of tyrosinase-related protein 2 as a tumor rejection antigen for the B16 melanoma. J Exp Med. 1997;185:453–9. doi: 10.1084/jem.185.3.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cox AL, Skipper J, Chen Y, Henderson RA, Darrow TL, Shabanowitz J, et al. Identification of a peptide recognized by five melanoma-specific human cytotoxic T cell lines. Science. 1994;264:716–9. doi: 10.1126/science.7513441. [DOI] [PubMed] [Google Scholar]
- 52.Bakker AB, Schreurs MW, de Boer AJ, Kawakami Y, Rosenberg SA, Adema GJ, et al. Melanocyte lineage-specific antigen gp100 is recognized by melanoma-derived tumor-infiltrating lymphocytes. J Exp Med. 1994;179:1005–9. doi: 10.1084/jem.179.3.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bowne WB, Srinivasan R, Wolchok JD, Hawkins WG, Blachere NE, Dyall R, et al. Coupling and uncoupling of tumor immunity and autoimmunity. J Exp Med. 1999;190:1717–22. doi: 10.1084/jem.190.11.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Overwijk WW, Lee DS, Surman DR, Irvine KR, Touloukian CE, Chan CC, et al. Vaccination with a recombinant vaccinia virus encoding a “self” antigen induces autoimmune vitiligo and tumor cell destruction in mice: requirement for CD4(+) T lymphocytes. Proc Natl Acad Sci U S A. 1999;96:2982–7. doi: 10.1073/pnas.96.6.2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Morgan DJ, Kreuwel HT, Fleck S, Levitsky HI, Pardoll DM, Sherman LA. Activation of low avidity CTL specific for a self epitope results in tumor rejection but not autoimmunity. J Immunol. 1998;160:643–51. [PubMed] [Google Scholar]
- 56.Colella TA, Bullock TN, Russell LB, Mullins DW, Overwijk WW, Luckey CJ, et al. Self-tolerance to the murine homologue of a tyrosinase-derived melanoma antigen: implications for tumor immunotherapy. J Exp Med. 2000;191:1221–32. doi: 10.1084/jem.191.7.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nichols LA, Chen Y, Colella TA, Bennett CL, Clausen BE, Engelhard VH. Deletional self-tolerance to a melanocyte/melanoma antigen derived from tyrosinase is mediated by a radio-resistant cell in peripheral and mesenteric lymph nodes. J Immunol. 2007;179:993–1003. doi: 10.4049/jimmunol.179.2.993. [DOI] [PubMed] [Google Scholar]
- 58.Hu J, Kindsvogel W, Busby S, Bailey MC, Shi YY, Greenberg PD. An evaluation of the potential to use tumor-associated antigens as targets for antitumor T cell therapy using transgenic mice expressing a retroviral tumor antigen in normal lymphoid tissues. J Exp Med. 1993;177:1681–90. doi: 10.1084/jem.177.6.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Staveley-O’Carroll K, Sotomayor E, Montgomery J, Borrello I, Hwang L, Fein S, et al. Induction of antigen-specific T cell anergy: An early event in the course of tumor progression. Proc Natl Acad Sci U S A. 1998;95:1178–83. doi: 10.1073/pnas.95.3.1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shrikant P, Khoruts A, Mescher MF. CTLA-4 blockade reverses CD8+ T cell tolerance to tumor by a CD4+ T cell- and IL-2-dependent mechanism. Immunity. 1999;11:483–93. doi: 10.1016/S1074-7613(00)80123-5. [DOI] [PubMed] [Google Scholar]
- 61.Schell TD, Knowles BB, Tevethia SS. Sequential loss of cytotoxic T lymphocyte responses to simian virus 40 large T antigen epitopes in T antigen transgenic mice developing osteosarcomas. Cancer Res. 2000;60:3002–12. [PubMed] [Google Scholar]
- 62.Willimsky G, Blankenstein T. Sporadic immunogenic tumours avoid destruction by inducing T-cell tolerance. Nature. 2005;437:141–6. doi: 10.1038/nature03954. [DOI] [PubMed] [Google Scholar]
- 63.Sotomayor EM, Borrello I, Rattis FM, Cuenca AG, Abrams J, Staveley-O’Carroll K, et al. Cross-presentation of tumor antigens by bone marrow-derived antigen-presenting cells is the dominant mechanism in the induction of T-cell tolerance during B-cell lymphoma progression. Blood. 2001;98:1070–7. doi: 10.1182/blood.V98.4.1070. [DOI] [PubMed] [Google Scholar]
- 64.Mihalyo MA, Hagymasi AT, Slaiby AM, Nevius EE, Adler AJ. Dendritic cells program non-immunogenic prostate-specific T cell responses beginning at early stages of prostate tumorigenesis. Prostate. 2007;67:536–46. doi: 10.1002/pros.20549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nguyen LT, Elford AR, Murakami K, Garza KM, Schoenberger SP, Odermatt B, et al. Tumor growth enhances cross-presentation leading to limited T cell activation without tolerance. J Exp Med. 2002;195:423–35. doi: 10.1084/jem.20010032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hanson HL, Donermeyer DL, Ikeda H, White JM, Shankaran V, Old LJ, et al. Eradication of established tumors by CD8+ T cell adoptive immunotherapy. Immunity. 2000;13:265–76. doi: 10.1016/S1074-7613(00)00026-1. [DOI] [PubMed] [Google Scholar]
- 67.Ochsenbein AF, Sierro S, Odermatt B, Pericin M, Karrer U, Hermans J, et al. Roles of tumour localization, second signals and cross priming in cytotoxic T-cell induction. Nature. 2001;411:1058–64. doi: 10.1038/35082583. [DOI] [PubMed] [Google Scholar]
- 68.Spiotto MT, Yu P, Rowley DA, Nishimura MI, Meredith SC, Gajewski TF, et al. Increasing tumor antigen expression overcomes “ignorance” to solid tumors via crosspresentation by bone marrow-derived stromal cells. Immunity. 2002;17:737–47. doi: 10.1016/S1074-7613(02)00480-6. [DOI] [PubMed] [Google Scholar]
- 69.Basu S, Binder RJ, Suto R, Anderson KM, Srivastava PK. Necrotic but not apoptotic cell death releases heat shock proteins, which deliver a partial maturation signal to dendritic cells and activate the NF-kappa B pathway. Int Immunol. 2000;12:1539–46. doi: 10.1093/intimm/12.11.1539. [DOI] [PubMed] [Google Scholar]
- 70.Shi Y, Evans JE, Rock KL. Molecular identification of a danger signal that alerts the immune system to dying cells. Nature. 2003;425:516–21. doi: 10.1038/nature01991. [DOI] [PubMed] [Google Scholar]
- 71.Apetoh L, Ghiringhelli F, Tesniere A, Obeid M, Ortiz C, Criollo A, et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med. 2007;13:1050–9. doi: 10.1038/nm1622. [DOI] [PubMed] [Google Scholar]
- 72.Pardoll D. Does the immune system see tumors as foreign or self? Annu Rev Immunol. 2003;21:807–39. doi: 10.1146/annurev.immunol.21.120601.141135. [DOI] [PubMed] [Google Scholar]
- 73.Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol. 2002;3:991–8. doi: 10.1038/ni1102-991. [DOI] [PubMed] [Google Scholar]
- 74.Zhou G, Lu Z, McCadden JD, Levitsky HI, Marson AL. Reciprocal changes in tumor antigenicity and antigen-specific T cell function during tumor progression. J Exp Med. 2004;200:1581–92. doi: 10.1084/jem.20041240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lee PP, Yee C, Savage PA, Fong L, Brockstedt D, Weber JS, et al. Characterization of circulating T cells specific for tumor-associated antigens in melanoma patients. Nat Med. 1999;5:677–85. doi: 10.1038/9525. [DOI] [PubMed] [Google Scholar]
- 76.Cella M, Scheidegger D, Palmer-Lehmann K, Lane P, Lanzavecchia A, Alber G. Ligation of CD40 on dendritic cells triggers production of high levels of interleukin-12 and enhances T cell stimulatory capacity: T-T help via APC activation. J Exp Med. 1996;184:747–52. doi: 10.1084/jem.184.2.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bennett SR, Carbone FR, Karamalis F, Flavell RA, Miller JF, Heath WR. Help for cytotoxic-T-cell responses is mediated by CD40 signalling. Nature. 1998;393:478–80. doi: 10.1038/30996. [DOI] [PubMed] [Google Scholar]
- 78.Schoenberger SP, Toes RE, van der Voort EI, Offringa R, Melief CJ. T-cell help for cytotoxic T lymphocytes is mediated by CD40-CD40L interactions. Nature. 1998;393:480–3. doi: 10.1038/31002. [DOI] [PubMed] [Google Scholar]
- 79.Ridge JP, Di Rosa F, Matzinger P. A conditioned dendritic cell can be a temporal bridge between a CD4+ T-helper and a T-killer cell. Nature. 1998;393:474–8. doi: 10.1038/30989. [DOI] [PubMed] [Google Scholar]
- 80.Maxwell JR, Campbell JD, Kim CH, Vella AT. CD40 activation boosts T cell immunity in vivo by enhancing T cell clonal expansion and delaying peripheral T cell deletion. J Immunol. 1999;162:2024–34. [PubMed] [Google Scholar]
- 81.Sotomayor EM, Borrello I, Tubb E, Rattis FM, Bien H, Lu Z, et al. Conversion of tumor-specific CD4+ T-cell tolerance to T-cell priming through in vivo ligation of CD40. Nat Med. 1999;5:780–7. doi: 10.1038/10503. [DOI] [PubMed] [Google Scholar]
- 82.Diehl L, den Boer AT, Schoenberger SP, van der Voort EI, Schumacher TN, Melief CJ, et al. CD40 activation in vivo overcomes peptide-induced peripheral cytotoxic T-lymphocyte tolerance and augments anti-tumor vaccine efficacy. Nat Med. 1999;5:774–9. doi: 10.1038/10495. [DOI] [PubMed] [Google Scholar]
- 83.Mallett S, Fossum S, Barclay AN. Characterization of the MRC OX40 antigen of activated CD4 positive T lymphocytes--a molecule related to nerve growth factor receptor. EMBO J. 1990;9:1063–8. doi: 10.1002/j.1460-2075.1990.tb08211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gramaglia I, Weinberg AD, Lemon M, Croft M. Ox-40 ligand: a potent costimulatory molecule for sustaining primary CD4 T cell responses. J Immunol. 1998;161:6510–7. [PubMed] [Google Scholar]
- 85.Murata K, Ishii N, Takano H, Miura S, Ndhlovu LC, Nose M, et al. Impairment of antigen-presenting cell function in mice lacking expression of OX40 ligand. J Exp Med. 2000;191:365–74. doi: 10.1084/jem.191.2.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Flynn S, Toellner KM, Raykundalia C, Goodall M, Lane P. CD4 T cell cytokine differentiation: the B cell activation molecule, OX40 ligand, instructs CD4 T cells to express interleukin 4 and upregulates expression of the chemokine receptor, Blr-1. J Exp Med. 1998;188:297–304. doi: 10.1084/jem.188.2.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rogers PR, Song J, Gramaglia I, Killeen N, Croft M. OX40 promotes Bcl-xL and Bcl-2 expression and is essential for long-term survival of CD4 T cells. Immunity. 2001;15:445–55. doi: 10.1016/S1074-7613(01)00191-1. [DOI] [PubMed] [Google Scholar]
- 88.So T, Song J, Sugie K, Altman A, Croft M. Signals from OX40 regulate nuclear factor of activated T cells c1 and T cell helper 2 lineage commitment. Proc Natl Acad Sci U S A. 2006;103:3740–5. doi: 10.1073/pnas.0600205103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Maxwell JR, Weinberg A, Prell RA, Vella AT. Danger and OX40 receptor signaling synergize to enhance memory T cell survival by inhibiting peripheral deletion. J Immunol. 2000;164:107–12. doi: 10.4049/jimmunol.164.1.107. [DOI] [PubMed] [Google Scholar]
- 90.Weatherill AR, Maxwell JR, Takahashi C, Weinberg AD, Vella AT. OX40 ligation enhances cell cycle turnover of Ag-activated CD4 T cells in vivo. Cell Immunol. 2001;209:63–75. doi: 10.1006/cimm.2001.1783. [DOI] [PubMed] [Google Scholar]
- 91.Lathrop SK, Huddleston CA, Dullforce PA, Montfort MJ, Weinberg AD, Parker DC. A signal through OX40 (CD134) allows anergic, autoreactive T cells to acquire effector cell functions. J Immunol. 2004;172:6735–43. doi: 10.4049/jimmunol.172.11.6735. [DOI] [PubMed] [Google Scholar]
- 92.Huddleston CA, Weinberg AD, Parker DC. OX40 (CD134) engagement drives differentiation of CD4+ T cells to effector cells. Eur J Immunol. 2006;36:1093–103. doi: 10.1002/eji.200535637. [DOI] [PubMed] [Google Scholar]
- 93.Redmond WL, Gough MJ, Charbonneau B, Ratliff TL, Weinberg AD. Defects in the acquisition of CD8 T cell effector function after priming with tumor or soluble antigen can be overcome by the addition of an OX40 agonist. J Immunol. 2007;179:7244–53. doi: 10.4049/jimmunol.179.11.7244. [DOI] [PubMed] [Google Scholar]
- 94.Bandyopadhyay S, Long M, Qui HZ, Hagymasi AT, Slaiby AM, Mihalyo MA, et al. Self-antigen prevents CD8 T cell effector differentiation by CD134 and CD137 dual costimulation. J Immunol. 2008;181:7728–37. doi: 10.4049/jimmunol.181.11.7728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Weinberg AD, Rivera MM, Prell R, Morris A, Ramstad T, Vetto JT, et al. Engagement of the OX-40 receptor in vivo enhances antitumor immunity. J Immunol. 2000;164:2160–9. doi: 10.4049/jimmunol.164.4.2160. [DOI] [PubMed] [Google Scholar]
- 96.Melero I, Shuford WW, Newby SA, Aruffo A, Ledbetter JA, Hellström KE, et al. Monoclonal antibodies against the 4-1BB T-cell activation molecule eradicate established tumors. Nat Med. 1997;3:682–5. doi: 10.1038/nm0697-682. [DOI] [PubMed] [Google Scholar]
- 97.Roberts DJ, Franklin NA, Kingeter LM, Yagita H, Tutt AL, Glennie MJ, et al. Control of established melanoma by CD27 stimulation is associated with enhanced effector function and persistence, and reduced PD-1 expression of tumor infiltrating CD8(+) T cells. J Immunother. 2010;33:769–79. doi: 10.1097/CJI.0b013e3181ee238f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Cohen AD, Diab A, Perales MA, Wolchok JD, Rizzuto G, Merghoub T, et al. Agonist anti-GITR antibody enhances vaccine-induced CD8(+) T-cell responses and tumor immunity. Cancer Res. 2006;66:4904–12. doi: 10.1158/0008-5472.CAN-05-2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Weinberg AD, Morris NP, Kovacsovics-Bankowski M, Urba WJ, Curti BD. Science gone translational: the OX40 agonist story. Immunol Rev. 2011;244:218–31. doi: 10.1111/j.1600-065X.2011.01069.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Snell LM, Lin GH, McPherson AJ, Moraes TJ, Watts TH. T-cell intrinsic effects of GITR and 4-1BB during viral infection and cancer immunotherapy. Immunol Rev. 2011;244:197–217. doi: 10.1111/j.1600-065X.2011.01063.x. [DOI] [PubMed] [Google Scholar]
- 101.Aggarwal BB. Signalling pathways of the TNF superfamily: a double-edged sword. Nat Rev Immunol. 2003;3:745–56. doi: 10.1038/nri1184. [DOI] [PubMed] [Google Scholar]
- 102.Kopf M, Ruedl C, Schmitz N, Gallimore A, Lefrang K, Ecabert B, et al. OX40-deficient mice are defective in Th cell proliferation but are competent in generating B cell and CTL Responses after virus infection. Immunity. 1999;11:699–708. doi: 10.1016/S1074-7613(00)80144-2. [DOI] [PubMed] [Google Scholar]
- 103.Gramaglia I, Jember A, Pippig SD, Weinberg AD, Killeen N, Croft M. The OX40 costimulatory receptor determines the development of CD4 memory by regulating primary clonal expansion. J Immunol. 2000;165:3043–50. doi: 10.4049/jimmunol.165.6.3043. [DOI] [PubMed] [Google Scholar]
- 104.Dawicki W, Bertram EM, Sharpe AH, Watts TH. 4-1BB and OX40 act independently to facilitate robust CD8 and CD4 recall responses. J Immunol. 2004;173:5944–51. doi: 10.4049/jimmunol.173.10.5944. [DOI] [PubMed] [Google Scholar]
- 105.Shuford WW, Klussman K, Tritchler DD, Loo DT, Chalupny J, Siadak AW, et al. 4-1BB costimulatory signals preferentially induce CD8+ T cell proliferation and lead to the amplification in vivo of cytotoxic T cell responses. J Exp Med. 1997;186:47–55. doi: 10.1084/jem.186.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Pollok KE, Kim Y-J, Zhou Z, Hurtado J, Kim KK, Pickard RT, et al. Inducible T cell antigen 4-1BB. Analysis of expression and function. J Immunol. 1993;150:771–81. [PubMed] [Google Scholar]
- 107.Takahashi C, Mittler RS, Vella AT. Cutting edge: 4-1BB is a bona fide CD8 T cell survival signal. J Immunol. 1999;162:5037–40. [PubMed] [Google Scholar]
- 108.Dawicki W, Watts TH. Expression and function of 4-1BB during CD4 versus CD8 T cell responses in vivo. Eur J Immunol. 2004;34:743–51. doi: 10.1002/eji.200324278. [DOI] [PubMed] [Google Scholar]
- 109.Tewalt EF, Cohen JN, Rouhani SJ, Guidi CJ, Qiao H, Fahl SP, et al. Lymphatic endothelial cells induce tolerance via PD-L1 and lack of costimulation leading to high-level PD-1 expression on CD8 T cells. Blood. 2012;120:4772–82. doi: 10.1182/blood-2012-04-427013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Melero I, Johnston JV, Shufford WW, Mittler RS, Chen L. NK1.1 cells express 4-1BB (CDw137) costimulatory molecule and are required for tumor immunity elicited by anti-4-1BB monoclonal antibodies. Cell Immunol. 1998;190:167–72. doi: 10.1006/cimm.1998.1396. [DOI] [PubMed] [Google Scholar]
- 111.Futagawa T, Akiba H, Kodama T, Takeda K, Hosoda Y, Yagita H, et al. Expression and function of 4-1BB and 4-1BB ligand on murine dendritic cells. Int Immunol. 2002;14:275–86. doi: 10.1093/intimm/14.3.275. [DOI] [PubMed] [Google Scholar]
- 112.Kwon B, Lee HW, Kwon BS. New insights into the role of 4-1BB in immune responses: beyond CD8+ T cells. Trends Immunol. 2002;23:378–80. doi: 10.1016/S1471-4906(02)02263-9. [DOI] [PubMed] [Google Scholar]
- 113.Pauly S, Broll K, Wittmann M, Giegerich G, Schwarz H. CD137 is expressed by follicular dendritic cells and costimulates B lymphocyte activation in germinal centers. J Leukoc Biol. 2002;72:35–42. [PubMed] [Google Scholar]
- 114.Myers LM, Vella AT. Interfacing T-cell effector and regulatory function through CD137 (4-1BB) co-stimulation. Trends Immunol. 2005;26:440–6. doi: 10.1016/j.it.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 115.Hildebrand JM, Yi Z, Buchta CM, Poovassery J, Stunz LL, Bishop GA. Roles of tumor necrosis factor receptor associated factor 3 (TRAF3) and TRAF5 in immune cell functions. Immunol Rev. 2011;244:55–74. doi: 10.1111/j.1600-065X.2011.01055.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Stamenkovic I, Clark EA, Seed B. A B-lymphocyte activation molecule related to the nerve growth factor receptor and induced by cytokines in carcinomas. EMBO J. 1989;8:1403–10. doi: 10.1002/j.1460-2075.1989.tb03521.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Alderson MR, Armitage RJ, Tough TW, Strockbine L, Fanslow WC, Spriggs MK. CD40 expression by human monocytes: regulation by cytokines and activation of monocytes by the ligand for CD40. J Exp Med. 1993;178:669–74. doi: 10.1084/jem.178.2.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Inaba K, Witmer-Pack M, Inaba M, Hathcock KS, Sakuta H, Azuma M, et al. The tissue distribution of the B7-2 costimulator in mice: abundant expression on dendritic cells in situ and during maturation in vitro. J Exp Med. 1994;180:1849–60. doi: 10.1084/jem.180.5.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Vu MD, Xiao X, Gao W, Degauque N, Chen M, Kroemer A, et al. OX40 costimulation turns off Foxp3+ Tregs. Blood. 2007;110:2501–10. doi: 10.1182/blood-2007-01-070748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Uno T, Takeda K, Kojima Y, Yoshizawa H, Akiba H, Mittler RS, et al. Eradication of established tumors in mice by a combination antibody-based therapy. Nat Med. 2006;12:693–8. doi: 10.1038/nm1405. [DOI] [PubMed] [Google Scholar]
- 121.Lee SJ, Myers L, Muralimohan G, Dai J, Qiao Y, Li Z, et al. 4-1BB and OX40 dual costimulation synergistically stimulate primary specific CD8 T cells for robust effector function. J Immunol. 2004;173:3002–12. doi: 10.4049/jimmunol.173.5.3002. [DOI] [PubMed] [Google Scholar]
- 122.Munks MW, Mourich DV, Mittler RS, Weinberg AD, Hill AB. 4-1BB and OX40 stimulation enhance CD8 and CD4 T-cell responses to a DNA prime, poxvirus boost vaccine. Immunology. 2004;112:559–66. doi: 10.1111/j.1365-2567.2004.01917.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Cuadros C, Dominguez AL, Lollini PL, Croft M, Mittler RS, Borgström P, et al. Vaccination with dendritic cells pulsed with apoptotic tumors in combination with anti-OX40 and anti-4-1BB monoclonal antibodies induces T cell-mediated protective immunity in Her-2/neu transgenic mice. Int J Cancer. 2005;116:934–43. doi: 10.1002/ijc.21098. [DOI] [PubMed] [Google Scholar]
- 124.Gray JC, French RR, James S, Al-Shamkhani A, Johnson PW, Glennie MJ. Optimising anti-tumour CD8 T-cell responses using combinations of immunomodulatory antibodies. Eur J Immunol. 2008;38:2499–511. doi: 10.1002/eji.200838208. [DOI] [PubMed] [Google Scholar]
- 125.Lee SJ, Rossi RJ, Lee SK, Croft M, Kwon BS, Mittler RS, et al. CD134 Costimulation Couples the CD137 Pathway to Induce Production of Supereffector CD8 T Cells That Become IL-7 Dependent. J Immunol. 2007;179:2203–14. doi: 10.4049/jimmunol.179.4.2203. [DOI] [PubMed] [Google Scholar]
- 126.Qui HZ, Hagymasi AT, Bandyopadhyay S, St Rose MC, Ramanarasimhaiah R, Ménoret A, et al. CD134 plus CD137 dual costimulation induces Eomesodermin in CD4 T cells to program cytotoxic Th1 differentiation. J Immunol. 2011;187:3555–64. doi: 10.4049/jimmunol.1101244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Murphy KM, Reiner SL. The lineage decisions of helper T cells. Nat Rev Immunol. 2002;2:933–44. doi: 10.1038/nri954. [DOI] [PubMed] [Google Scholar]
- 128.Glimcher LH, Townsend MJ, Sullivan BM, Lord GM. Recent developments in the transcriptional regulation of cytolytic effector cells. Nat Rev Immunol. 2004;4:900–11. doi: 10.1038/nri1490. [DOI] [PubMed] [Google Scholar]
- 129.Misko IS, Pope JH, Hütter R, Soszynski TD, Kane RG. HLA-DR-antigen-associated restriction of EBV-specific cytotoxic T-cell colonies. Int J Cancer. 1984;33:239–43. doi: 10.1002/ijc.2910330212. [DOI] [PubMed] [Google Scholar]
- 130.Williams NS, Engelhard VH. Identification of a population of CD4+ CTL that utilizes a perforin- rather than a Fas ligand-dependent cytotoxic mechanism. J Immunol. 1996;156:153–9. [PubMed] [Google Scholar]
- 131.Appay V, Zaunders JJ, Papagno L, Sutton J, Jaramillo A, Waters A, et al. Characterization of CD4(+) CTLs ex vivo. J Immunol. 2002;168:5954–8. doi: 10.4049/jimmunol.168.11.5954. [DOI] [PubMed] [Google Scholar]
- 132.Jellison ER, Kim SK, Welsh RM. Cutting edge: MHC class II-restricted killing in vivo during viral infection. J Immunol. 2005;174:614–8. doi: 10.4049/jimmunol.174.2.614. [DOI] [PubMed] [Google Scholar]
- 133.Swain SL, McKinstry KK, Strutt TM. Expanding roles for CD4⁺ T cells in immunity to viruses. Nat Rev Immunol. 2012;12:136–48. doi: 10.1038/nri3152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Topalian SL, Rivoltini L, Mancini M, Markus NR, Robbins PF, Kawakami Y, et al. Human CD4+ T cells specifically recognize a shared melanoma-associated antigen encoded by the tyrosinase gene. Proc Natl Acad Sci U S A. 1994;91:9461–5. doi: 10.1073/pnas.91.20.9461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ferrone S, Marincola FM. Loss of HLA class I antigens by melanoma cells: molecular mechanisms, functional significance and clinical relevance. Immunol Today. 1995;16:487–94. doi: 10.1016/0167-5699(95)80033-6. [DOI] [PubMed] [Google Scholar]
- 136.Xie Y, Akpinarli A, Maris C, Hipkiss EL, Lane M, Kwon EK, et al. Naive tumor-specific CD4(+) T cells differentiated in vivo eradicate established melanoma. J Exp Med. 2010;207:651–67. doi: 10.1084/jem.20091921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Quezada SA, Simpson TR, Peggs KS, Merghoub T, Vider J, Fan X, et al. Tumor-reactive CD4(+) T cells develop cytotoxic activity and eradicate large established melanoma after transfer into lymphopenic hosts. J Exp Med. 2010;207:637–50. doi: 10.1084/jem.20091918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Pearce EL, Mullen AC, Martins GA, Krawczyk CM, Hutchins AS, Zediak VP, et al. Control of effector CD8+ T cell function by the transcription factor Eomesodermin. Science. 2003;302:1041–3. doi: 10.1126/science.1090148. [DOI] [PubMed] [Google Scholar]
- 139.Intlekofer AM, Takemoto N, Wherry EJ, Longworth SA, Northrup JT, Palanivel VR, et al. Effector and memory CD8+ T cell fate coupled by T-bet and eomesodermin. Nat Immunol. 2005;6:1236–44. doi: 10.1038/ni1268. [DOI] [PubMed] [Google Scholar]
- 140.Maeurer MJ, Gollin SM, Martin D, Swaney W, Bryant J, Castelli C, et al. Tumor escape from immune recognition: lethal recurrent melanoma in a patient associated with downregulation of the peptide transporter protein TAP-1 and loss of expression of the immunodominant MART-1/Melan-A antigen. J Clin Invest. 1996;98:1633–41. doi: 10.1172/JCI118958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Jäger E, Ringhoffer M, Altmannsberger M, Arand M, Karbach J, Jäger D, et al. Immunoselection in vivo: independent loss of MHC class I and melanocyte differentiation antigen expression in metastatic melanoma. Int J Cancer. 1997;71:142–7. doi: 10.1002/(SICI)1097-0215(19970410)71:2<142::AID-IJC3>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 142.Gottschalk S, Ng CY, Perez M, Smith CA, Sample C, Brenner MK, et al. An Epstein-Barr virus deletion mutant associated with fatal lymphoproliferative disease unresponsive to therapy with virus-specific CTLs. Blood. 2001;97:835–43. doi: 10.1182/blood.V97.4.835. [DOI] [PubMed] [Google Scholar]
- 143.Yee C, Thompson JA, Byrd D, Riddell SR, Roche P, Celis E, et al. Adoptive T cell therapy using antigen-specific CD8+ T cell clones for the treatment of patients with metastatic melanoma: in vivo persistence, migration, and antitumor effect of transferred T cells. Proc Natl Acad Sci U S A. 2002;99:16168–73. doi: 10.1073/pnas.242600099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Spiotto MT, Rowley DA, Schreiber H. Bystander elimination of antigen loss variants in established tumors. Nat Med. 2004;10:294–8. doi: 10.1038/nm999. [DOI] [PubMed] [Google Scholar]
- 145.Ngoi SM, St Rose MC, Menoret AM, Smith DE, Tovey MG, Adler AJ, et al. Presensitizing with a Toll-like receptor 3 ligand impairs CD8 T-cell effector differentiation and IL-33 responsiveness. Proc Natl Acad Sci U S A. 2012;109:10486–91. doi: 10.1073/pnas.1202607109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Lüthi AU, Cullen SP, McNeela EA, Duriez PJ, Afonina IS, Sheridan C, et al. Suppression of interleukin-33 bioactivity through proteolysis by apoptotic caspases. Immunity. 2009;31:84–98. doi: 10.1016/j.immuni.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 147.Bonilla WV, Fröhlich A, Senn K, Kallert S, Fernandez M, Johnson S, et al. The alarmin interleukin-33 drives protective antiviral CD8⁺ T cell responses. Science. 2012;335:984–9. doi: 10.1126/science.1215418. [DOI] [PubMed] [Google Scholar]
- 148.Lacy MQ, Jacobus S, Blood EA, Kay NE, Rajkumar SV, Greipp PR. Phase II study of interleukin-12 for treatment of plateau phase multiple myeloma (E1A96): a trial of the Eastern Cooperative Oncology Group. Leuk Res. 2009;33:1485–9. doi: 10.1016/j.leukres.2009.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Rosenberg SA, White DE. Vitiligo in patients with melanoma: normal tissue antigens can be targets for cancer immunotherapy. J Immunother Emphasis Tumor Immunol. 1996;19:81–4. doi: 10.1097/00002371-199601000-00009. [DOI] [PubMed] [Google Scholar]
- 150.Yee C, Thompson JA, Roche P, Byrd DR, Lee PP, Piepkorn M, et al. Melanocyte destruction after antigen-specific immunotherapy of melanoma: direct evidence of t cell-mediated vitiligo. J Exp Med. 2000;192:1637–44. doi: 10.1084/jem.192.11.1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Beck KE, Blansfield JA, Tran KQ, Feldman AL, Hughes MS, Royal RE, et al. Enterocolitis in patients with cancer after antibody blockade of cytotoxic T-lymphocyte-associated antigen 4. J Clin Oncol. 2006;24:2283–9. doi: 10.1200/JCO.2005.04.5716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Liakou CI, Kamat A, Tang DN, Chen H, Sun J, Troncoso P, et al. CTLA-4 blockade increases IFNgamma-producing CD4+ICOShi cells to shift the ratio of effector to regulatory T cells in cancer patients. Proc Natl Acad Sci U S A. 2008;105:14987–92. doi: 10.1073/pnas.0806075105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Fadel F, El Karoui K, Knebelmann B. Anti-CTLA4 antibody-induced lupus nephritis. N Engl J Med. 2009;361:211–2. doi: 10.1056/NEJMc0904283. [DOI] [PubMed] [Google Scholar]
- 154.Carthon BC, Wolchok JD, Yuan J, Kamat A, Ng Tang DS, Sun J, et al. Preoperative CTLA-4 blockade: tolerability and immune monitoring in the setting of a presurgical clinical trial. Clin Cancer Res. 2010;16:2861–71. doi: 10.1158/1078-0432.CCR-10-0569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–23. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Suntharalingam G, Perry MR, Ward S, Brett SJ, Castello-Cortes A, Brunner MD, et al. Cytokine storm in a phase 1 trial of the anti-CD28 monoclonal antibody TGN1412. N Engl J Med. 2006;355:1018–28. doi: 10.1056/NEJMoa063842. [DOI] [PubMed] [Google Scholar]
- 157.Ascierto PA, Simeone E, Sznol M, Fu YX, Melero I. Clinical experiences with anti-CD137 and anti-PD1 therapeutic antibodies. Semin Oncol. 2010;37:508–16. doi: 10.1053/j.seminoncol.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 158.Van Assche G, Rutgeerts P. Anti-TNF agents in Crohn’s disease. Expert Opin Investig Drugs. 2000;9:103–11. doi: 10.1517/13543784.9.1.103. [DOI] [PubMed] [Google Scholar]
- 159.Kober J, Leitner J, Klauser C, Woitek R, Majdic O, Stöckl J, et al. The capacity of the TNF family members 4-1BBL, OX40L, CD70, GITRL, CD30L and LIGHT to costimulate human T cells. Eur J Immunol. 2008;38:2678–88. doi: 10.1002/eji.200838250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Ma BY, Mikolajczak SA, Danesh A, Hosiawa KA, Cameron CM, Takaori-Kondo A, et al. The expression and the regulatory role of OX40 and 4-1BB heterodimer in activated human T cells. Blood. 2005;106:2002–10. doi: 10.1182/blood-2004-04-1622. [DOI] [PubMed] [Google Scholar]
- 161.Kober J, Leitner J, Klauser C, Woitek R, Majdic O, Stöckl J, et al. The capacity of the TNF family members 4-1BBL, OX40L, CD70, GITRL, CD30L and LIGHT to costimulate human T cells. Eur J Immunol. 2008;38:2678–88. doi: 10.1002/eji.200838250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Serghides L, Bukczynski J, Wen T, Wang C, Routy JP, Boulassel MR, et al. Evaluation of OX40 ligand as a costimulator of human antiviral memory CD8 T cell responses: comparison with B7.1 and 4-1BBL. J Immunol. 2005;175:6368–77. doi: 10.4049/jimmunol.175.10.6368. [DOI] [PubMed] [Google Scholar]
- 163.Sawyers CL. Making progress through molecular attacks on cancer. Cold Spring Harb Symp Quant Biol. 2005;70:479–82. doi: 10.1101/sqb.2005.70.034. [DOI] [PubMed] [Google Scholar]
- 164.Nazarian R, Shi H, Wang Q, Kong X, Koya RC, Lee H, et al. Melanomas acquire resistance to B-RAF(V600E) inhibition by RTK or N-RAS upregulation. Nature. 2010;468:973–7. doi: 10.1038/nature09626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Villanueva J, Vultur A, Lee JT, Somasundaram R, Fukunaga-Kalabis M, Cipolla AK, et al. Acquired resistance to BRAF inhibitors mediated by a RAF kinase switch in melanoma can be overcome by cotargeting MEK and IGF-1R/PI3K. Cancer Cell. 2010;18:683–95. doi: 10.1016/j.ccr.2010.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Johannessen CM, Boehm JS, Kim SY, Thomas SR, Wardwell L, Johnson LA, et al. COT drives resistance to RAF inhibition through MAP kinase pathway reactivation. Nature. 2010;468:968–72. doi: 10.1038/nature09627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455–65. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Hurwitz AA, Yu TF, Leach DR, Allison JP. CTLA-4 blockade synergizes with tumor-derived granulocyte-macrophage colony-stimulating factor for treatment of an experimental mammary carcinoma. Proc Natl Acad Sci U S A. 1998;95:10067–71. doi: 10.1073/pnas.95.17.10067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Hurwitz AA, Foster BA, Kwon ED, Truong T, Choi EM, Greenberg NM, et al. Combination immunotherapy of primary prostate cancer in a transgenic mouse model using CTLA-4 blockade. Cancer Res. 2000;60:2444–8. [PubMed] [Google Scholar]
- 170.Curran MA, Kim M, Montalvo W, Al-Shamkhani A, Allison JP. Combination CTLA-4 blockade and 4-1BB activation enhances tumor rejection by increasing T-cell infiltration, proliferation, and cytokine production. PLoS One. 2011;6:e19499. doi: 10.1371/journal.pone.0019499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Sierro SR, Donda A, Perret R, Guillaume P, Yagita H, Levy F, et al. Combination of lentivector immunization and low-dose chemotherapy or PD-1/PD-L1 blocking primes self-reactive T cells and induces anti-tumor immunity. Eur J Immunol. 2011;41:2217–28. doi: 10.1002/eji.201041235. [DOI] [PubMed] [Google Scholar]
- 172.Borrello I, Sotomayor EM, Rattis FM, Cooke SK, Gu L, Levitsky HI. Sustaining the graft-versus-tumor effect through posttransplant immunization with granulocyte-macrophage colony-stimulating factor (GM-CSF)-producing tumor vaccines. Blood. 2000;95:3011–9. [PubMed] [Google Scholar]
- 173.Nigam A, Yacavone RF, Zahurak ML, Johns CM, Pardoll DM, Piantadosi S, et al. Immunomodulatory properties of antineoplastic drugs administered in conjunction with GM-CSF-secreting cancer cell vaccines. Int J Oncol. 1998;12:161–70. doi: 10.3892/ijo.12.1.161. [DOI] [PubMed] [Google Scholar]
- 174.Machiels JP, Reilly RT, Emens LA, Ercolini AM, Lei RY, Weintraub D, et al. Cyclophosphamide, doxorubicin, and paclitaxel enhance the antitumor immune response of granulocyte/macrophage-colony stimulating factor-secreting whole-cell vaccines in HER-2/neu tolerized mice. Cancer Res. 2001;61:3689–97. [PubMed] [Google Scholar]
- 175.Dummer W, Niethammer AG, Baccala R, Lawson BR, Wagner N, Reisfeld RA, et al. T cell homeostatic proliferation elicits effective antitumor autoimmunity. J Clin Invest. 2002;110:185–92. doi: 10.1172/JCI15175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Hu HM, Poehlein CH, Urba WJ, Fox BA. Development of antitumor immune responses in reconstituted lymphopenic hosts. Cancer Res. 2002;62:3914–9. [PubMed] [Google Scholar]
- 177.Schiavoni G, Mattei F, Di Pucchio T, Santini SM, Bracci L, Belardelli F, et al. Cyclophosphamide induces type I interferon and augments the number of CD44(hi) T lymphocytes in mice: implications for strategies of chemoimmunotherapy of cancer. Blood. 2000;95:2024–30. [PubMed] [Google Scholar]
- 178.Nowak AK, Lake RA, Marzo AL, Scott B, Heath WR, Collins EJ, et al. Induction of tumor cell apoptosis in vivo increases tumor antigen cross-presentation, cross-priming rather than cross-tolerizing host tumor-specific CD8 T cells. J Immunol. 2003;170:4905–13. doi: 10.4049/jimmunol.170.10.4905. [DOI] [PubMed] [Google Scholar]
- 179.Casares N, Pequignot MO, Tesniere A, Ghiringhelli F, Roux S, Chaput N, et al. Caspase-dependent immunogenicity of doxorubicin-induced tumor cell death. J Exp Med. 2005;202:1691–701. doi: 10.1084/jem.20050915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Hirschhorn-Cymerman D, Rizzuto GA, Merghoub T, Cohen AD, Avogadri F, Lesokhin AM, et al. OX40 engagement and chemotherapy combination provides potent antitumor immunity with concomitant regulatory T cell apoptosis. J Exp Med. 2009;206:1103–16. doi: 10.1084/jem.20082205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Hirschhorn-Cymerman D, Budhu S, Kitano S, Liu C, Zhao F, Zhong H, et al. Induction of tumoricidal function in CD4+ T cells is associated with concomitant memory and terminally differentiated phenotype. J Exp Med. 2012;209:2113–26. doi: 10.1084/jem.20120532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 183.Denmeade SR, Isaacs JT. A history of prostate cancer treatment. Nat Rev Cancer. 2002;2:389–96. doi: 10.1038/nrc801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Hakimi JM, Rondinelli RH, Schoenberg MP, Barrack ER. Androgen-receptor gene structure and function in prostate cancer. World J Urol. 1996;14:329–37. doi: 10.1007/BF00184606. [DOI] [PubMed] [Google Scholar]
- 185.Chen CD, Welsbie DS, Tran C, Baek SH, Chen R, Vessella R, et al. Molecular determinants of resistance to antiandrogen therapy. Nat Med. 2004;10:33–9. doi: 10.1038/nm972. [DOI] [PubMed] [Google Scholar]
- 186.Drake CG, Doody AD, Mihalyo MA, Huang CT, Kelleher E, Ravi S, et al. Androgen ablation mitigates tolerance to a prostate/prostate cancer-restricted antigen. Cancer Cell. 2005;7:239–49. doi: 10.1016/j.ccr.2005.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Antonarakis ES, Drake CG. Combining immunological and androgen-directed approaches: an emerging concept in prostate cancer immunotherapy. Curr Opin Oncol. 2012;24:258–65. doi: 10.1097/CCO.0b013e32835205a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Sutherland JS, Goldberg GL, Hammett MV, Uldrich AP, Berzins SP, Heng TS, et al. Activation of thymic regeneration in mice and humans following androgen blockade. J Immunol. 2005;175:2741–53. doi: 10.4049/jimmunol.175.4.2741. [DOI] [PubMed] [Google Scholar]
- 189.Bansal-Pakala P, Jember AG, Croft M. Signaling through OX40 (CD134) breaks peripheral T-cell tolerance. Nat Med. 2001;7:907–12. doi: 10.1038/90942. [DOI] [PubMed] [Google Scholar]
- 190.Redmond WL, Gough MJ, Weinberg AD. Ligation of the OX40 co-stimulatory receptor reverses self-Ag and tumor-induced CD8 T-cell anergy in vivo. Eur J Immunol. 2009;39:2184–94. doi: 10.1002/eji.200939348. [DOI] [PMC free article] [PubMed] [Google Scholar]