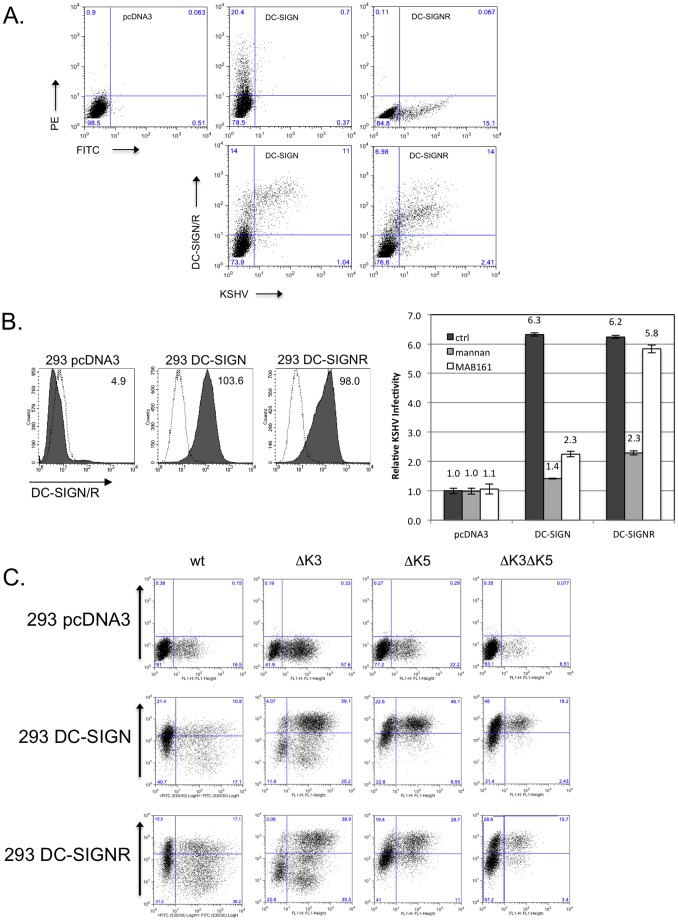
Figure 1. Infectivity of KSHV is enhanced in the presence of DC-SIGN and DC-SIGNR.
A) 293T cells were transfected with empty pcDNA3 vector or expression constructs for DC-SIGN or DC-SIGNR. After 24 hours, cells were infected with 20 µl Bac16ΔK3ΔK5 or left uninfected as controls. Cells were harvested after additional 24 hours and surface stained with a DC-SIGN/R antibody (H-200) and analyzed by flow cytometry. Top three panels show transfected cells stained for DC-SIGN/R followed by PE- (DC-SIGN), FITC- (DC-SIGNR) or both (vector) conjugated secondary antibodies. Bottom panels shows KSHV infection of 293T cells transiently expressing DC-SIGN or DC-SIGNR. B, left panels) 293 cell lines stably expressing a vector construct, DC-SIGN or DC-SIGNR were fluorescently stained for surface expression of DC-SIGN or DC-SIGNR. The mean channel fluorescence is indicated in the upper right hand corner. Open histograms – secondary antibody alone; shaded histograms – DC-SIGN or DC-SIGNR staining. B, right panel) 293 pcDNA3, DC-SIGN or DC-SIGNR stable cell lines were pre-incubated with a control antibody (anti-ICAM1, 7 µg/ml), with mannan (100 µg/ml) or a monoclonal antibody specific for DC-SIGN (MAB161; 7 µg/ml) for 30 minutes on ice. These cells were then infected with wild type KSHV (Bac16 or rKSHV.219) at an MOI of 0.01. After 72 hours cells were harvested and evaluated for infection by flow cytometry measuring GFP expression. Infection rates were normalized to 293 pcDNA3 cells treated with the control antibody. The fold increase in relative infectivity is indicated. Data are representative of four independent experiments with two performed in triplicate. C) 293 pcDNA3, DC-SIGN or DC-SIGNR stable lines were infected with 50 µl of concentrated Bac16 wildtype (wt), or mutants with deletion of K3 only (ΔK3), K5 only (ΔK5), or deletion of both K3 and K5 (ΔK3ΔK5) as indicated. At 72 hours post-infection, the cells were stained for surface expression of DC-SIGN, DC-SIGNR or MHC class I. GFP fluorescence was used as a marker for infection. MHC I staining is shown for infected 293 DC-SIGNR cells. Inset numbers indicate percentage of cells in each quadrant.