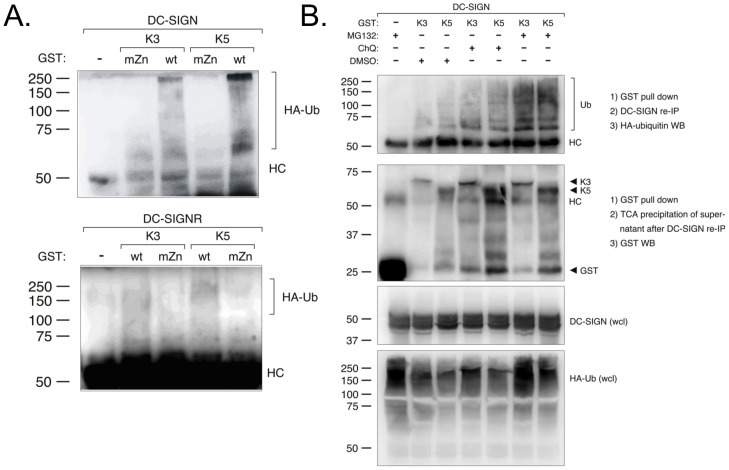
Figure 7. DC-SIGN and DC-SIGNR are ubiquitylated and degraded in a proteasomal- and lysosomal-dependent pathway by K3 and K5.
A) 293T cells were co-transfected with either 1.75 µg DC-SIGN (top panels) or 1.75 µg DC-SIGNR (bottom panels), 0.5 ug HA-tagged ubiquitin, and 1.75 µg wild-type or RING-CH mutant constructs of GST-tagged K3 or K5, as indicated. At 36–48 hours post-transfection, cells were collected and lysed. Pull-down (PD) was done using glutathione-sepharose beads, followed by SDS-PAGE and immunoblotting analysis. The precipitated proteins were probed with anti-HA antibodies. B) 293T cells were transiently transfected as in panel A. At 36–48 hours post-transfection cells were treated with chloroquine (ChQ ), MG132, or DMSO (solvent), as indicated. Cell lysates were then subjected to pull-down with glutathione beads followed by immunoprecipitation of DC-SIGN. Precipitated proteins were then subjected to western blot with an anti-HA antibody. Supernatants from the immunoprecipitation were subjected to TCA precipitation and then western blot with an anti-GST antibody. As additional controls, whole cell lysates were subjected to western blot with antibodies against DC-SIGN or HA-tagged ubiquitin. Data is representative of multiple experiments.