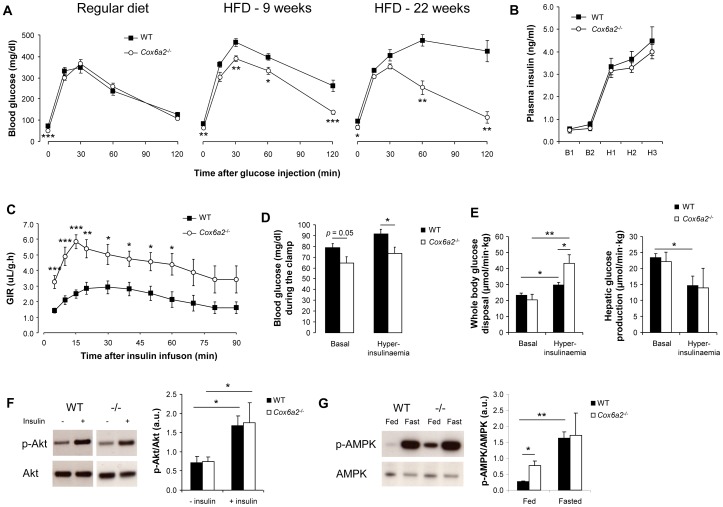
Figure 3. Increased glucose tolerance and insulin sensitivity in Cox6a2 −/− mice is associated with constitutive activation of AMPK.
(A) After a 16–18 h fast, mice were injected with 2.5 mg/g BW glucose and blood glucose levels were monitored for 2 h (n = 7–9 for RD, n = 4–11 for 9 weeks of HFD, n = 3–4 for 22 weeks of HFD). Note that WT animals become progressively glucose intolerant when receiving HFD, whereas Cox6a2 −/− mice are completely protected against HFD-induced glucose intolerance, (B) Hyperinsulinemic, euglycemic clamps were performed on mice (21 weeks old) fed a HFD for 15 weeks (n = 6–8). Plasma insulin levels before (10 min: B1; 0 min: B2) and after (70 min: H1; 80 min: H2; 90 min: H3) insulin infusion, (C) The glucose infusion rate (GIR) was monitored for 90 min after administration of a hyperinsulinemic solution via the tail vein, (D) Blood glucose levels before insulin infusion (basal) and at the end of the clamp (hyperinsulinaemia), (E) Whole body glucose disposal (left) and hepatic glucose production (right) were measured during the basal period and under hyperinsulinemic conditions, (F) Western blot analysis of insulin-stimulated phosphorylation of Akt in soleus muscle of regular diet fed mice. No difference was observed between fasted (18 h) WT and Cox6a2 −/− mice (n = 3), (G) Western blot analysis of AMPK phosphorylation in response to fasting. Mice (n = 3) on a regular diet were either fed ad libitum or fasted overnight (18 h) before dissection of soleus muscles. *p<0.05, **p<0.01, ***p<0.001. In all panels, data represent mean ± SEM.