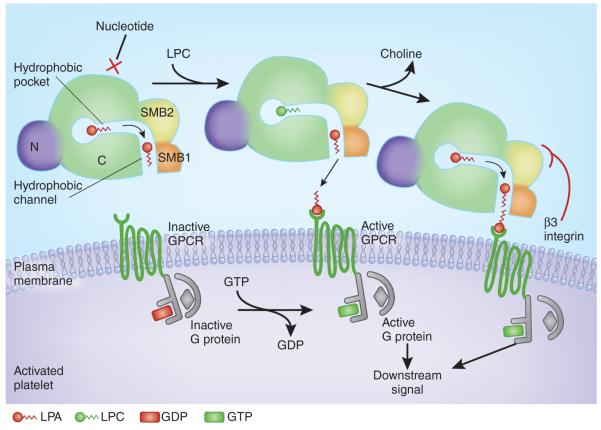
Figure 1.
Model for substrate recognition by ATX, LPA production and local receptor supersaturation. The surface of an activated platelet is shown, with the cartoon of a cross-section through the ATX protein and with domains labeled (C, catalytic domain; N, nuclease-like domain). Nucleotides cannot bind the active site when phospholipid substrates are engaged. A hydrophobic pocket in ATX accommodates LPC acyl chains and allows substrate discrimination. A channel between the catalytic site and SMB1 likely binds and shuttles LPA products before local delivery to the target receptor. Interaction of the SMB2 domain from ATX with a β3 integrin on the surface of the platelet may induce a conformational change in ATX that facilitates local release of LPA product at the cognate GPCR. This would achieve a high local specific concentration of product at the receptor site, effectively supersaturating the receptor relative to the rest of the platelet microenvironment and eliciting intracellular downstream signals. A flat surface at the hydrophobic channel entrance facilitates local interfacing with the target cell membrane.