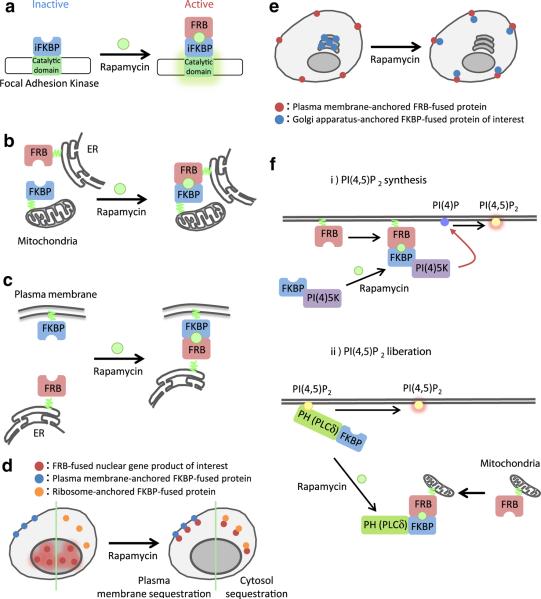
Fig. 3.
Further variations on the CID theme. a Kinase activation: Insertion of the iFKBP into the kinase domain of a protein can cause the protein to be catalytically inactive. Binding of the iFKBP to rapamycin and FRB induces a conformational change that restores kinase activity. b and c Membrane cross-linking: FRB and FKBP proteins are anchored to different cellular membranes. Addition of rapamycin leads to cross-linking of the membranes. d “Anchor-Away” technique: The POI is initially at its active location. Addition of rapamycin induces translocation to a different domain where is sequestered and inactive. e Inactive reservoir: POI is initially sequestered at a location where it is inactive (in this case the Golgi) rather than floating freely in the cytoplasm where it can have background activity. Addition of dimerizer induces translocation to the active site without membrane cross-linking. f Lipid synthesis versus lipid liberation: standard targeting of a PI(4) 5-kinase to plasma membrane induces formation of PI(4,5)P2, but also depletes the plasma membrane supply of PI(4)P. If instead the PI(4,5)P2 at the plasma membrane is initially “hidden” with a PH domain, which is then removed by sequestration to the mitochondrial membrane, the concentration of available PI(4,5)P2 at the plasma membrane is suddenly increased without affecting concentrations of other membrane lipids