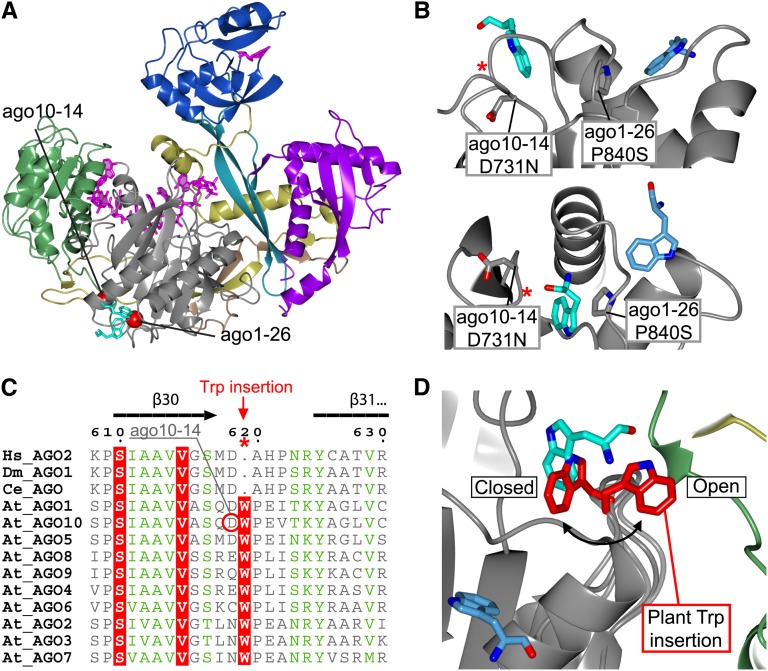
Figure 6.
Hs-Ago2 Trp Binding Pockets Are Likely to Be Conserved in Plants.
(A) Structural overview of Hs-Ago2 pointing out the close proximity between the ago10-14 and ago1-26 mutations and the ligand-bound Trp residues (cyan), suggesting a functional connection to the Trp binding sites.
(B) Top: Side view of the two Trp binding pockets. Pro-840 mutated in ago1-26 constitutes parts of one of the pockets (Trp; light blue), but the mutation could interfere with both pockets due to its central location (cyan and light blue). The ago10-14 D731N mutation shows how changing the nonconserved environment involved in Trp binding in Hs-Ago2 has an effect in plants. Bottom: Different view of top panel. The red asterisks indicate the location of the Trp insertion pointed out in (C).
(C) The primary sequence alignment of Human (Hs), Drosophila (Dm), C. elegans (Ce), and Arabidopsis AGOs reveals insertion of a conserved Trp in the loop connecting β30-β31 in plant AGOs.
(D) Backbone modeling of the At-AGO1 loop connecting β30-β31. The model shows the conformation of the plant-specific Trp (red) inserted at the Hs-Ago2 Trp binding site (cyan). The model suggests two possible conformations: one excluding additional Trp binding (closed) and one allowing binding of hydrophobic elements from an external source (open).