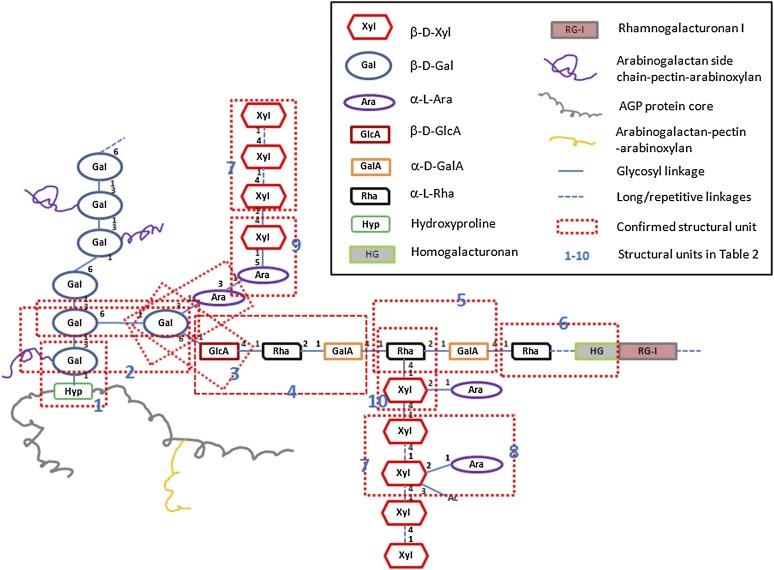
Figure 4.
Proposed Structural Model for APAP1.
RG-I is attached to type II AG through an α-d-GalA-1→2-α-l-Rha-1→4-β-d-GlcA-1→6-Gal structural unit (box 4). HG, with at least four to five GalA residues is embedded within RG-I (box 6). The length of flanking RG-I and HG domains is unknown. Arabinoxylan1 is attached to type II AG through a β-d-Xylp-1→5-α-l-Araf- linkage (box 9). However, only one out of three possible types of Ara residues (based on the common sizes of the arabinosyl side chains: Ara1, Ara2, and Ara3) for this attachment is shown in this model. Furthermore, some of the Xyl residues in arabinoxylan1 are arabinosylated at position 2. Arabinoxylan2 is attached to RG-I through a β-d-Xylp-1→4-α-l-Rha linkage (box 10). The AGP protein core in APAP1 is AGP57C encoded by At3g45230. Bolded blue numbers (1 to 10) represent the identified structural units as listed in Table 2. Based on the amount of Hyp and monosaccharides in YS1 and YS2, a given Hyp residue in At-AGP57C in YS1 is attached with, on average, ∼24 Gal, 45 Ara, 28 Xyl, seven Rha, and 13 GalA/GlcA, while in YS2, there are 11 Gal, 65 Ara, 65 Xyl, nine Rha, and 13 GalA/GlcA residues. Due to the heterogeneity of glycosylation, this proposed model reflects representative, and not exact, numbers and length of the AG-pectin-(arabino)xylan chains in APAP1.