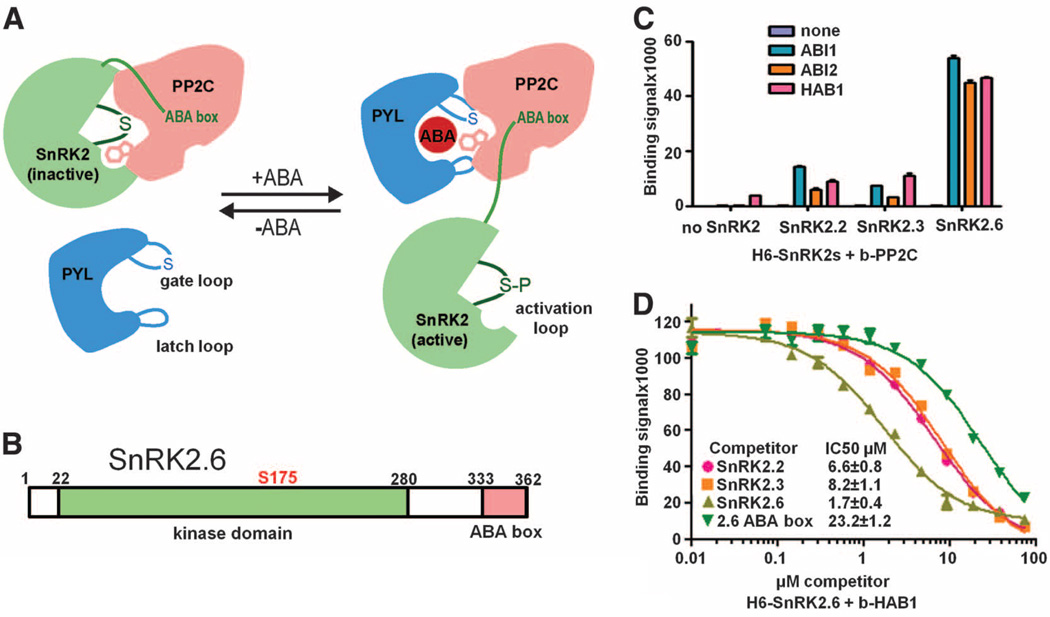
Fig. 1.
SnRK2–PP2C interactions. (A) Summary model for the interactions between SnRK2, PP2C, and the ABA receptor PYR/PYL/RCAR in ABA signaling. In the absence of ABA, PP2C binds to the SnRK2 kinase domain and inhibits the kinase activity by dephosphorylating the activation loop serine and blocking the catalytic cleft. In the presence of ABA, ABA-receptor complex binds to PP2C and inhibits PP2C’s catalytic activity by inserting the gate loop into the PP2C active cleft. PYL-mediated inhibition of PP2C allows activation of the kinase by activation loop autophosphorylation. The activated kinase then transmits the ABA signal by phosphorylating downstream factors. (B) Schematic presentation of the domain structure of SnRK2.6 with amino acid residue numbers shown on top. (C) Interactions of ABI1, ABI2, and HAB1 with SnRK2.2, 2.3, and 2.6. Binding of recombinant H6GST-SnRK2s to biotin-PP2Cs was determined by AlphaScreen assays (see fig. S2 and Materials and Methods). Error bars indicate SD (n = 3). (D) Inhibition of the interaction between H6GST-SnRK2.6 and biotin-HAB1 by untagged SnRK2s and SnRK2.6 ABA box peptide (amino acids 333 to 362). The IC50 values were derived from curve fitting. Error bars indicate SD (n = 3).