Fig 6.
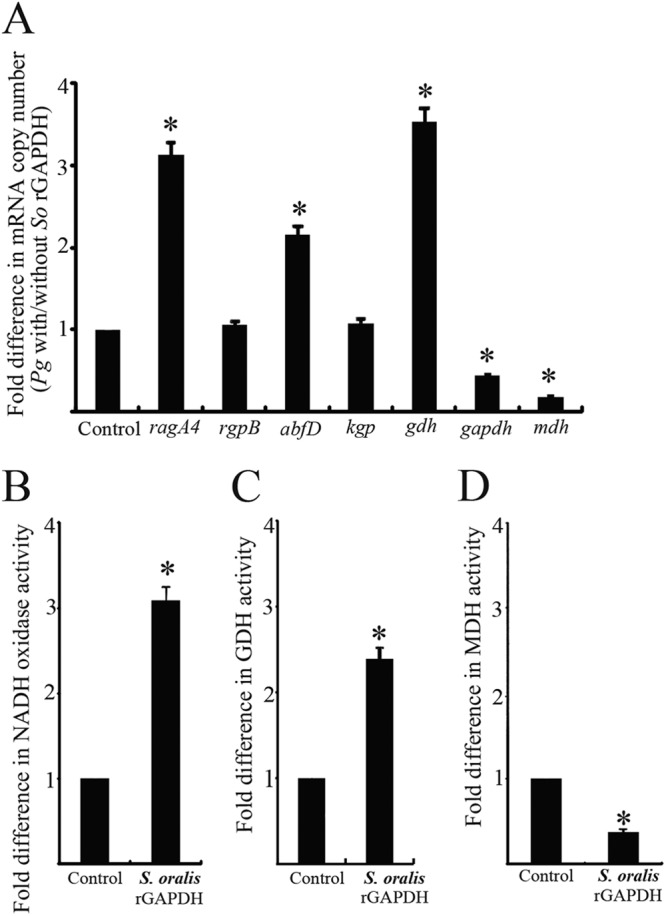
Modulation of P. gingivalis client protein expression by S. oralis rGAPDH. (A) Real-time quantitative RT-PCR of mRNA levels for genes involved in P. gingivalis client protein production and secretion. Transcript levels were normalized to 16S rRNA. P. gingivalis without S. oralis rGAPDH served as a control. The fold differences were expressed as targeted mRNA levels after exposure to S. oralis rGAPDH (5 μg) compared to the control. Data are means and standard deviations from three independent experiments performed in triplicate. An asterisk denotes a significant difference at P < 0.01 (t test) compared to the control. (B) Measurement of P. gingivalis cell surface NADH oxidase activity. NADH oxidase activity was measured at 25°C by monitoring the oxidation of β-NADH at 340 nm. (C) Measurement of P. gingivalis cell surface GDH activity. GDH activity was measured as the reduction of NAD by monitoring the oxidation of glutamate to α-ketoglutamate at 340 nm and 37°C. (D) Measurement of P. gingivalis cell surface MDH assays. MDH activity was measured by monitoring NADH at 340 nm and 37°C. P. gingivalis without S. oralis rGAPDH served as a control. The fold differences of NADH oxidase activity, GDH activity, and MDH activity were expressed as enzymatic activity after exposure to S. oralis rGAPDH (5 μg) compared to that of the control. Data are means and standard deviations from three independent experiments performed in triplicate. An asterisk denotes a significant difference at P < 0.01 (t test) compared to P. gingivalis alone.
