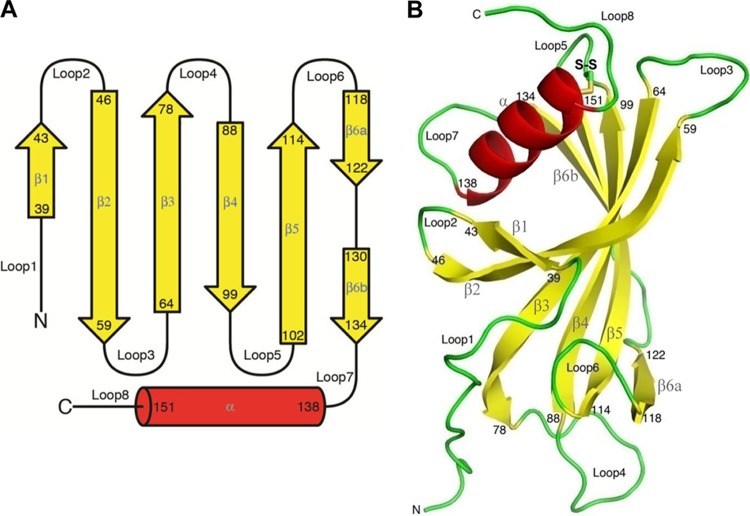
Fig 2.
Secondary-structure elements and the PE monomer. (A) PE 28 to 159 amino acids showing the secondary structure. In total, 6 β-strands, 8 loops, and one C-terminal helix exist. (B) In the monomer, 6 antiparallel β-strands form the β-sheet. A longer α-helix packs on the concave face of the sheet, where strands β1, β2, and β3 are curved around it. It is tethered to the β-sheet through a conserved disulfide bond between cysteines 99 and 148.