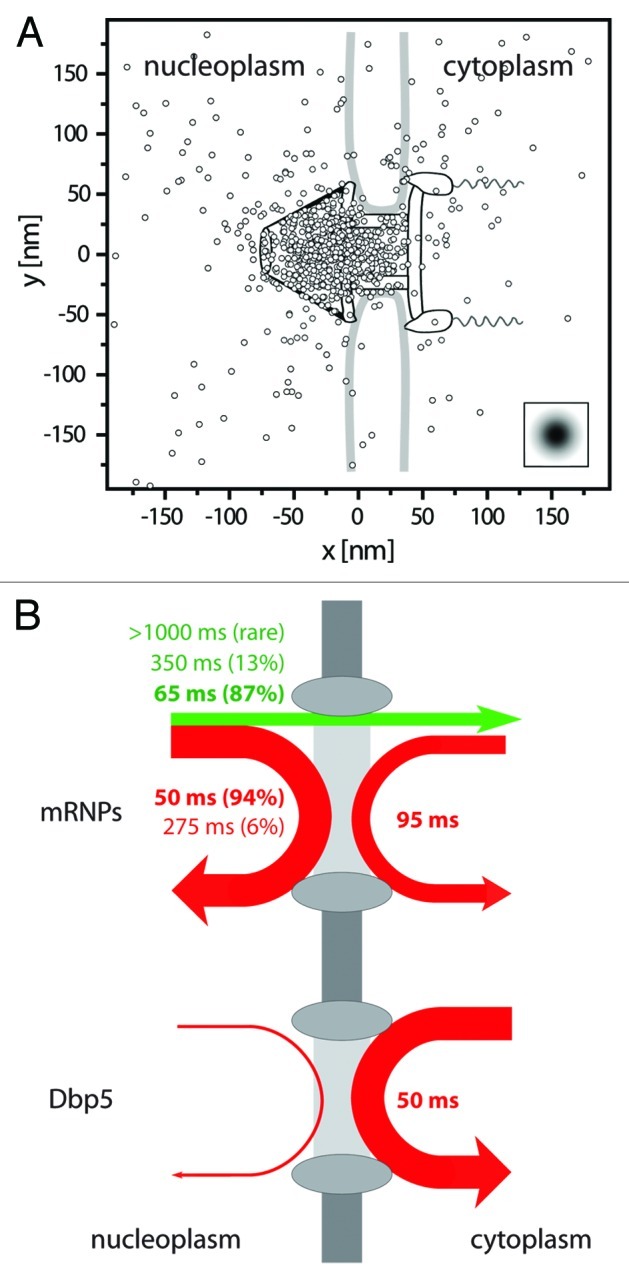
Figure 2. Binding sites and interaction durations with the nuclear pore complexes. (A) Distribution of binding sites of mRNP trajectories during export across the NPC. The figure shows a superposition of export trajectory positions with durations > 300 ms. The trajectories were aligned for an optimal overlay with the known NPC structure.30 The graphics shows the single positions superimposed onto the NPC scheme drawn to scale. The insert indicates the experimental single molecule localization precision. Figure according to Siebrasse et al.6 (B) Illustration of the interactions of mRNPs (labeled by hrp36) and the RNA helicase Dbp5 with the nuclear envelope. The line width indicates the relative occurrence of the respective process, and the color a successful (green) or unsuccessful (red) translocation. Upper graph: about 25% of the approaching mRNPs were successfully translocated across the nuclear envelope (straight green arrow), whereas 75% returned into the nucleus (red arrow). The numbers give the average interaction duration with the nuclear envelope, and the size of the relative fractions is reported in brackets. More than twice as many mRNPs encountered the nuclear envelope coming from nucleoplasm than from cytoplasm. Still, the latter events suggested that a fraction of mRNPs was still mobile in the cytoplasm, and was not immediately engaged in translation. Lower graph: most encounters of Dbp5 (91%) with the nuclear envelope began and ended in the cytoplasm, the interaction time was just a bit shorter than that of the majority of export events. Nuclear import of Dbp5 could not be observed at the used time resolution (20 ms), since protein import occurs fast, namely in 4 to 10 ms.17
