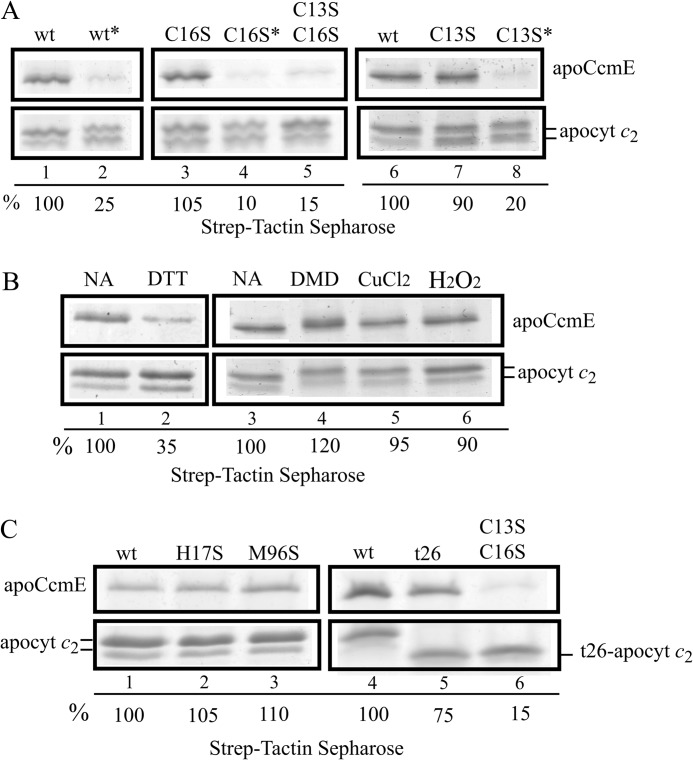
FIGURE 4.
Co-purification of apoCcmE with various apocytochrome c2 mutants. A, co-purifications of His-apoCcmE with wild type Strep-apocytochrome c2 (lanes 1 and 6), single C16S (lane 3), single C13S (lane 7), double C13S/C16S (lane 5) Cys mutants and their respective DTT reduced/iodoacetamide alkylated derivatives, wt* (lane 2), C16S* (lane 4), C13S* (lane 8) are shown. B, co-purification of His-apoCcmE with wild type Strep-apocytochrome c2 in the presence of 10 mm DTT (lane 2), 5 mm diamide (DMD; lane 4), 1 mm CuCl2 (lane 5), and 1 mm H2O2 (lane 6) are shown together with untreated samples (no addition (NA)), lanes 1 and 3). C, co-purification of His-apoCcmE with wild type Strep-apocytochrome c2 (lane 1 and 3), and H17S (lane 2), M96S (lane 3) heme iron axial ligand mutants and the t26-truncated derivative of Strep-apocytochrome c2 lacking its last 26-amino acid residues (lane 5) as well as its double cysteine mutant t26-C13S/C16S (lane 6) are shown. In panels A–C, the relative amount of His-apoCcmE co-purified with wild type Strep-apocytochrome c2 was taken as 100% for image quantification using Image J program and used for comparison with the amounts seen in the other lanes. All co-purification assays were done under the standard conditions and used Strep-Tactin-Sepharose resin, as described under “Experimental Procedures.” Panel A and B show the regions of the gel containing the two forms of apocytochrome c2 and the major monomeric form of CcmE for the sake of clarity.