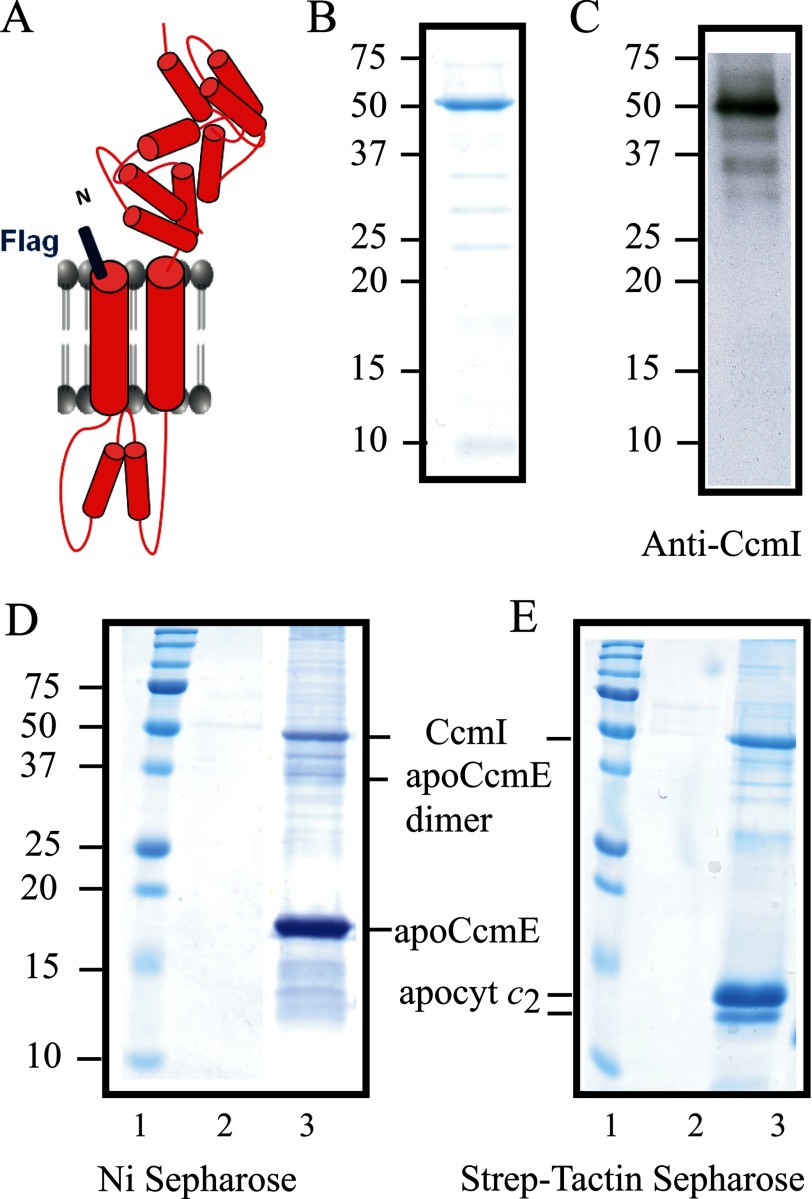
FIGURE 5.
R. capsulatus CcmI co-purifies with apoCcmE. A, a schematic representation of FLAG-CcmI shows its CcmI-1 domain with two transmembrane helices and its TPR containing periplasmic CcmI-2 domain. B, Coomassie Blue staining of purified FLAG-CcmI shows a single band centered at ∼50 kDa. C, an immunoblot of purified FLAG-CcmI using anti-CcmI antibodies shows mainly the 50-kDa band and upon overexposure several very faint bands corresponding to its degradation products. D, shown is co-purification of FLAG-CcmI with His-apoCcmE using Ni2+-Sepharose resin (lane 3). E, co-purification of FLAG-CcmI with Strep-apocytochrome c2 using a Strep-Tactin-Sepharose resin (lane 3) is shown. Lanes 2 show that FLAG-CcmI is not retained unspecifically in the Ni2+-Sepharose (panel D) and Strep-Tactin-Sepharose (panel E) columns.