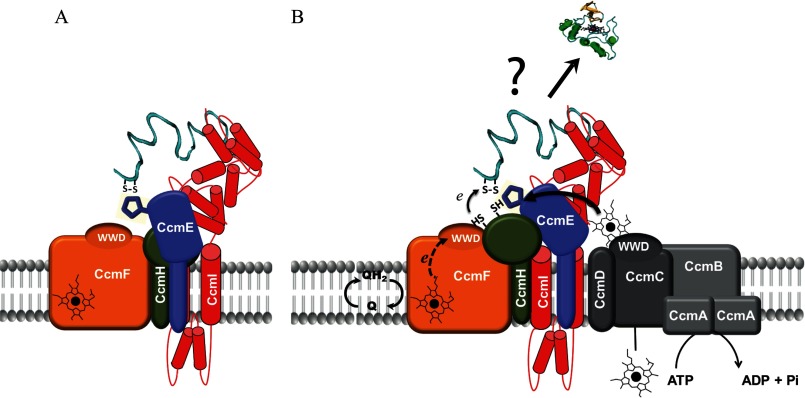
FIGURE 8.
Hypothetical models for apoCcmE interactions with an apocytochrome substrate and the CcmFHI heme ligation complex. A, the figure depicts apoCcmE interacting directly with apocytochrome c2 and the R. capsulatus heme ligation complex components CcmI and CcmH while interacting indirectly with CcmF. The model derives from the observations that apoCcmE forms a stable ternary complex with apocytochrome c and CcmI and co-purifies less readily with CcmF, inferring direct and indirect interactions, respectively. B, the figure depicts a putative large molecular entity composed of the CcmFHI-CcmE complex (as in panel A) associated with the heme translocation complex CcmABCD. If such a supercomplex exists, then conceivably the C-terminal portion of the unfolded apocytochrome c2 is captured via the periplasmic domain of CcmI of the CcmFHI core complex and interacts with apoCcmE to position its heme-binding site near CcmH so that heme-binding site disulfide bond reduction occurs if needed. When heme is translocated from the cytoplasm and becomes available at CcmC, then apoCcmE is converted to holoCcmE by interacting directly with CcmC and CcmD. After ATP hydrolysis by CcmA, holoCcmE becomes available to transfer heme to the apocytochrome c2, which remains trapped at the CcmFHI complex, with CcmF responsible for reduction of the heme iron in holoCcmE to facilitate heme ligation. It should be emphasized that the mechanism and order of the events described in panel B are hypothetical.