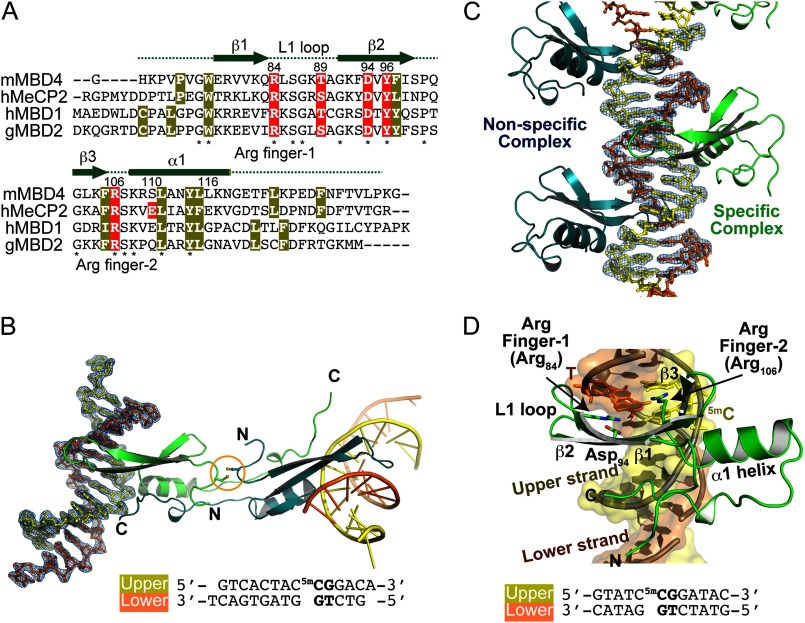
FIGURE 2.
Overall structure of MBDMBD4 complexed with DNA containing 5mCG/TG. A, sequence alignment of structurally known MBD domains; mouse MBD4 (mMBD4, 69–136 amino acids), human MeCP2 (hMeCP2, 88–167 amino acids), human MBD1 (hMBD1, 1–75 amino acids), and chicken MBD2 (gMBD2, 3–72 amino acids). Asterisks indicate the conserved residues among the MBD proteins. The hydrophobic core residues are highlighted in yellow. The residues highlighted in red are involved in recognition of methylated CpG base pairs. B and C, overall structures of MBDMBD4-5mCG/TG complexes. B, C2221 orthorhombic form; C, P1 triclinic form. The 2mFo − DFc electron density map for the DNA molecule contoured at 1.5 σ is shown in blue. The lower DNA strand containing a mismatched thymine is presented in orange, and the complementary upper strand is yellow. A disulfide bond linking two symmetry-related MBDs is indicated by an orange circle in B. In the P1 form, specific (green) and nonspecific (blue) MBDMBD4-DNA complexes are evident (C). D, specific binding of MBDMBD4 to 5mCG/TG. The triclinic P1 form structure of MBDMBD4 is shown as a green ribbon representation. Side chains of Arg-84, Arg-106, and Asp-94 are presented as stick models. The lower DNA strand containing a mismatched thymine is presented in orange, and the complementary upper strand is yellow. The 5mCG/TG base pairs are shown as stick models. The DNA sequences are indicated below in B and D.