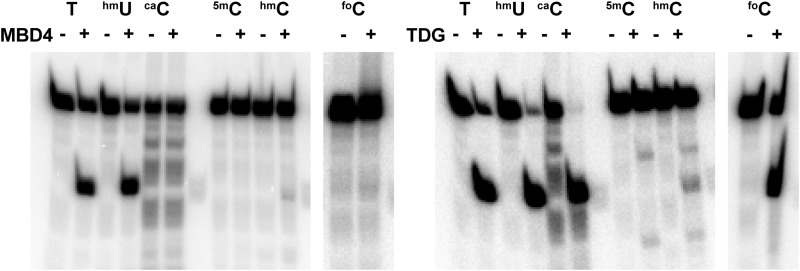
FIGURE 9.
Glycosylase activities of MBD4 and TDG. The glycosylase activity of the full-length MBD4 protein for mismatched, deamination, and/or oxidation products in the context of the 5mCG/5mCG sequence was assessed by NaOH cleavage of the resulting apyrimidinic site. We observed a significant digestion band for the strand containing either T or hmU in a mismatched wobble base. 5mC, hmC, foC, and caC bases, each of which forms canonical Watson-Crick base pairs, were not removed by MBD4, whereas, human TDG exhibited activity toward foC and caC in addition to T and hmU bases.