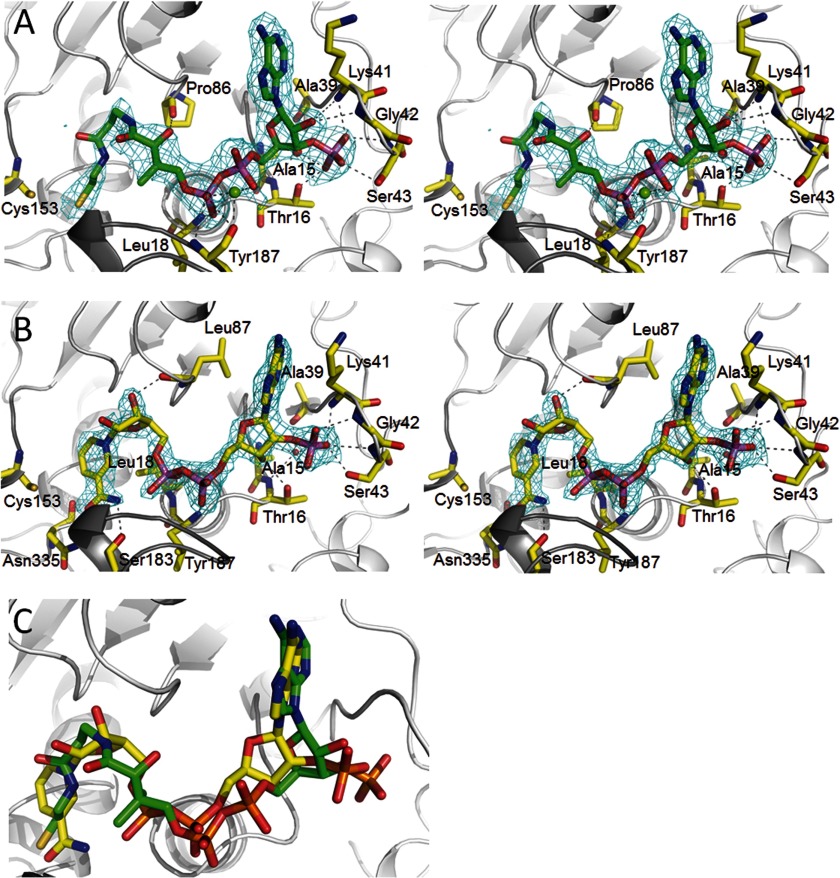
FIGURE 3.
Cofactor binding to MCR. A, NADP+ binding in stereo. NADP+ binds in a characteristic S-shaped conformation and is sandwiched between the loops of the C-terminal end of the β-sheet (light gray) and the cover loop (dark gray). Only residues forming hydrogen bonds to the polypeptide are shown. NADP+ is firmly anchored to the polypeptide at the 2′-ribose phosphate binding site. Proton donors comprise Lys41 NH, Gly42 NH, Ser43 NH, Ser43 OγH, and Thr16 Oγ1H as well as via a solvent molecule Ala39 O, Thr16 NH, and Ala15 NH. The 2Fobs − Fcalc electron density (cyan) is drawn at a contour level of 1.3σ. B, CoA binding in stereo. CoA is arranged in an S-shaped, rather compressed conformation (distance between α-phosphate O2A and β-cysteamine N4P, 8.7 Å) and embedded into the same binding site as NADP+. A metal ion seems to be positioned between the α- and β-phosphate and the Tyr187 carbonyl oxygens. The 2Fobs − Fcalc electron density (cyan) is drawn at a contour level of 1.5σ. C, superposition of NADP+ and CoA. The carbons of NADP+ are colored in yellow, and CoA carbons are in green. The distances between the β-phosphate of CoA and the α-phosphate of NADP+ and between their 3′- and 2′-ribose phosphates are ∼1.4 Å.