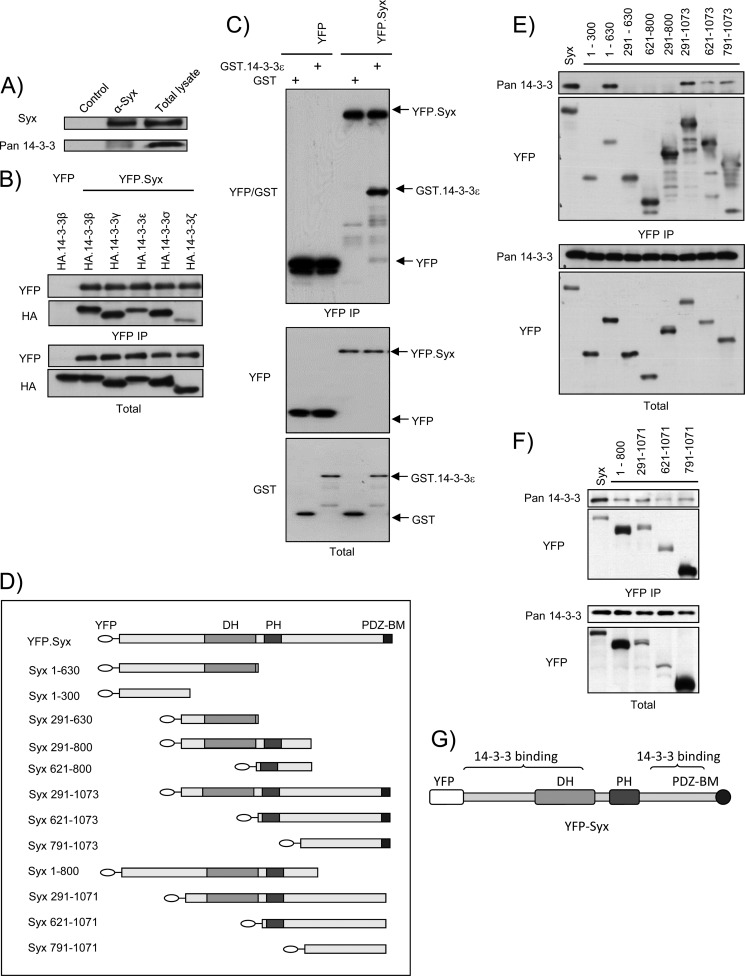
FIGURE 1.
Syx associates with 14-3-3 proteins at its termini. A, endogenous Syx co-immunoprecipitates with 14-3-3 proteins. Confluent HeLa cells were lysed and subjected to immunoprecipitation with unconjugated (control) or anti-Syx antibody-conjugated protein G beads. Total and immunoprecipitated cell lysates were analyzed by Western blotting. Pan anti-14-3-3 (K-19) antibody was used. B, YFP-Syx interacts with HA-14-3-3β, ϵ, γ, σ, and ζ. HeLa cells were co-transfected with plasmids encoding YFP or YFP-Syx and HA-14-3-3β, ϵ, γ, σ, or ζ. Cells were lysed and subjected to immunoprecipitation with anti-YFP antibody-conjugated protein G beads. Total and immunoprecipitated cell lysates were analyzed by Western blotting using anti-YFP and anti-HA antibodies. C, YFP-Syx binds GST-14-3-3ϵ in vitro. YFP or YFP-Syx was expressed in HeLa cells and immunoprecipitated with anti-YFP antibody-conjugated protein G beads; YFP and YFP-Syx-bound beads were subsequently incubated with GST or purified recombinant GST-14-3-3ϵ (see “Experimental Procedures”). Protein complexes were eluted and analyzed with Western blotting using anti-YFP and anti-GST antibodies. D, schematic domain structure depicts YFP-tagged murine Syx and Syx truncation variants. The first and last amino acids of each murine Syx fragment are shown as numbers in the corresponding labeling. E and F, 14-3-3 proteins bind two distinct regions of Syx. HeLa cells were transfected with the indicated constructs and subjected to immunoprecipitation (IP) with anti-YFP antibody-conjugated protein G beads. Co-immunoprecipitated 14-3-3 proteins were detected by immunoblotting with pan anti-14-3-3 (K-19) antibody. G, schematic diagram denotes regions of Syx where 14-3-3 proteins bind.