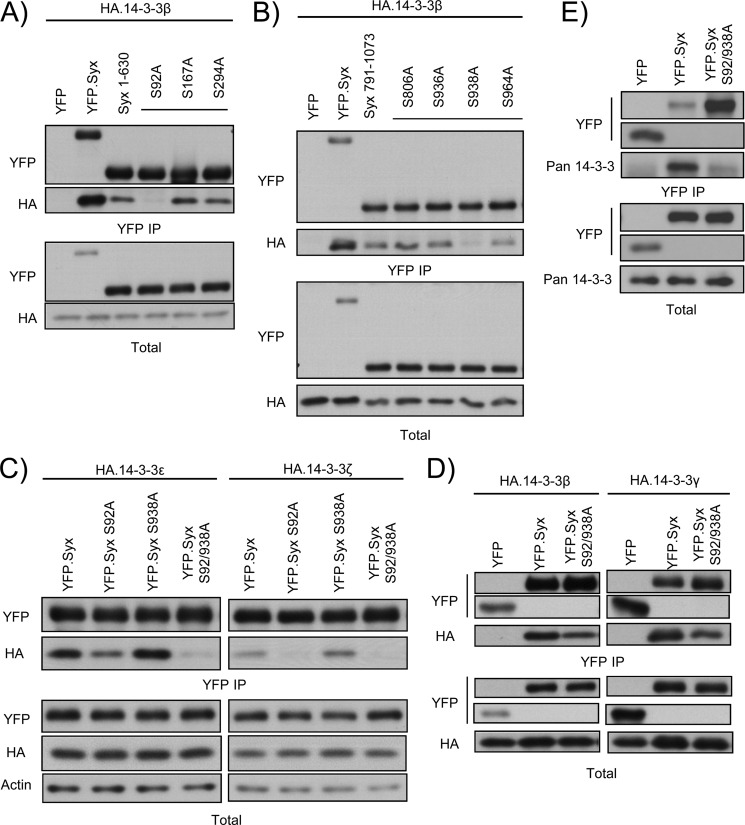
FIGURE 2.
Ser92 and Ser938 mediate 14-3-3 binding to Syx. A, Ser92 is a putative 14-3-3-binding residue. HeLa cells were co-transfected with plasmids encoding HA-14-3-3β and YFP, YFP-Syx, YFP-Syx(1–630), or one of three YFP-Syx(1–630) mutants in which a single serine was mutated to alanine. Cells were subjected to immunoprecipitation, and protein samples were analyzed by Western blotting. Only the replacement of Ser92 with alanine abolished the interaction between Syx(1–630) and 14-3-3β. B, Ser938 is a putative 14-3-3-binding residue. As in A, HeLa cells were co-transfected with plasmids encoding HA-14-3-3β and YFP, YFP-Syx, YFP-Syx(791–1073), or one of four YFP-Syx(791–1073) in which a single serine was mutated to alanine. Only the replacement of Ser938 with alanine abolished the interaction between Syx(791–1073) and 14-3-3β. Note that Ser806 was previously shown as a PKD-mediated phosphorylation target; mutating Ser806 to alanine, however, has no effect on the binding of 14-3-3β. C and D, Ser92 and Ser938 are essential residues for 14-3-3 interaction. HeLa cells were co-transfected with the indicated constructs and subjected to immunoprecipitation. Protein samples were analyzed by Western blot analysis. Mutating Ser92 and Ser938 on Syx either abolished or diminished its interaction with 14-3-3 isoforms. Note that mutation of Ser938 alone in C has no clear effect on 14-3-3 binding, but resulted in a decrease or absence of 14-3-3 co-immunoprecipitates when combined with S92A mutation. E, endogenous 14-3-3 proteins fail to co-immunoprecipitate with Syx S92A/S938A. HeLa cells were transfected with the indicated constructs and subjected to immunoprecipitation. Protein samples were analyzed by Western blotting. Mutating Ser92 and Ser938 on Syx significantly diminished its interaction with endogenous 14-3-3 proteins.