FIGURE 5.
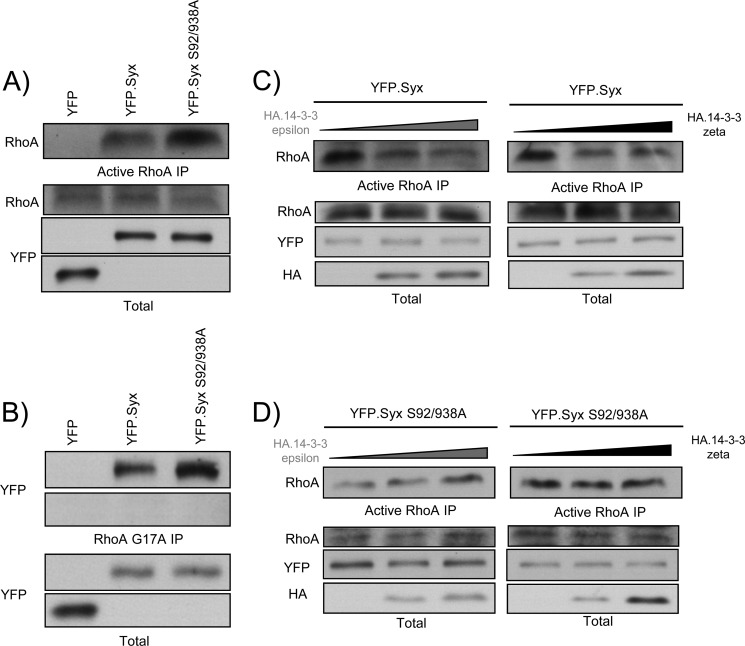
Binding of 14-3-3 proteins inhibits the nucleotide exchange activity of Syx. A, 14-3-3-uncoupled Syx has high GEF activity. HeLa cells were transfected with plasmids encoding YFP, YFP-Syx, or YFP-Syx S92A/S938A. Cells were lysed 24 h after transfection, and the supernatants were incubated with GST-fused Rhotekin-RBD beads to bind active RhoA. Total and pulled-down active RhoA were determined by SDS-PAGE and immunoblotting. B, Syx S92A/S938A is highly active. As in A, cells were transfected with the indicated constructs and lysed 24 h after transfection. The supernatants were incubated with RhoA G17A-conjugated glutathione beads to pull down active RhoGEFs. Total and pulled-down active Syx were analyzed by SDS-PAGE and immunoblotting. C, expression of 14-3-3ϵ and ζ inhibits Syx-induced RhoA activation. HeLa cells were co-transfected with YFP-Syx and an increasing amount (0, 1, or 2 μg, respectively) of HA-14-3-3ϵ or ζ. As in A, active RhoA pulldown assay was performed, and protein samples were analyzed by Western blotting. D, Syx S92A/S938A-induced RhoA activation is unaffected by the expression of 14-3-3ϵ or ζ. As in C, active RhoA was pulled down from lysates of HeLa cells co-expressing YFP-Syx S92A/S938A and an increasing amount (0, 1, or 2 μg, respectively) of HA-14-3-3ϵ or ζ. Total and active RhoA were determined by SDS-PAGE and immunoblotting.