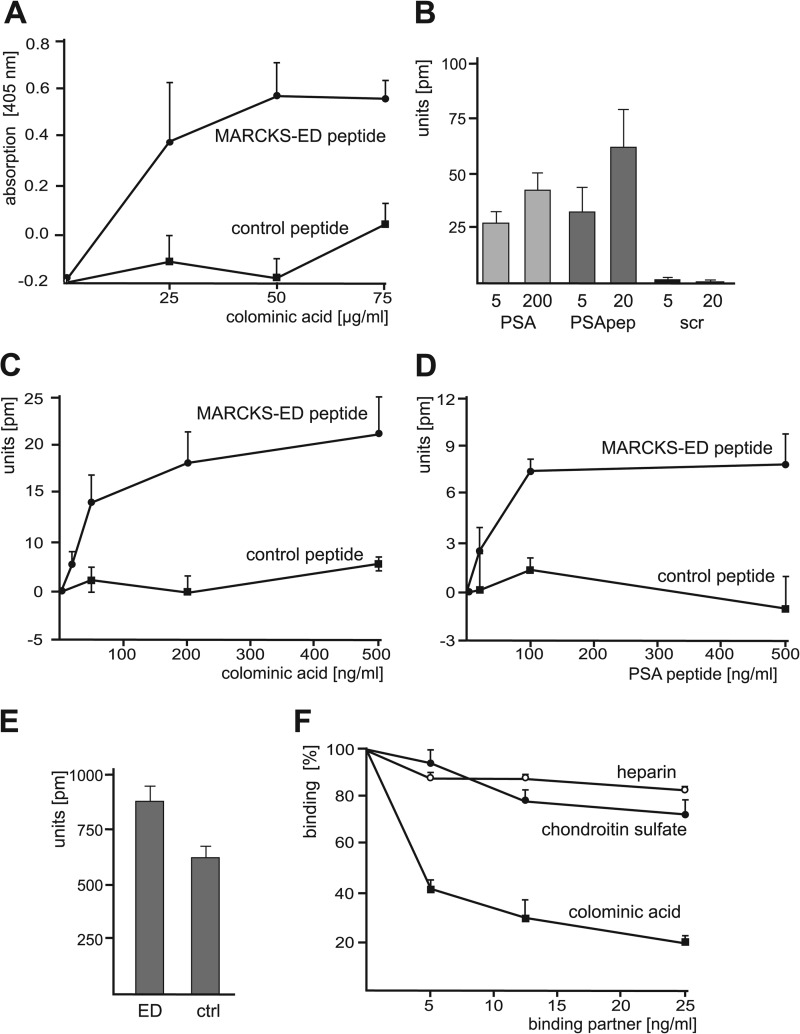
FIGURE 2.
Identification of a direct binding of PSA to the MARCKS effector domain. A, MARCKS-ED peptide and the corresponding control peptide as negative control were substrate-coated and incubated with increasing concentrations of colominic acid/PSA. The binding of colominic acid/PSA was detected by ELISA using PSA antibody and HRP-conjugated secondary antibodies. Mean values ± S.D. from three independent experiments carried out in triplicate are shown. B, MARCKS-ED and control peptide were substrate-coated and incubated with 5 or 200 ng/ml colominic acid/PSA (PSA), PSA-mimicking peptide (PSApep), or a scrambled version of the PSA-mimicking peptide (scr). C and D, substrate-coated MARCKS-ED peptide or control peptide was incubated with increasing amounts of colominic acid/PSA (C) or PSA-mimicking peptide (D). B—D, binding was determined by measuring the peak wavelength shifts in a label-free binding assay. E, coating efficiency of MARCKS-ED peptide (ED) or control peptide (ctrl) was determined by measuring the peak wavelength shifts. F, MARCKS-ED and control peptide were substrate-coated and incubated with PSA-mimicking peptide in the absence or presence of increasing concentrations of colominic acid/PSA, chondroitin sulfate, or heparin. Binding was determined by measuring the peak wavelength shifts. B–F, mean values ± S.D. from of two independent experiments carried out in triplicates are shown.