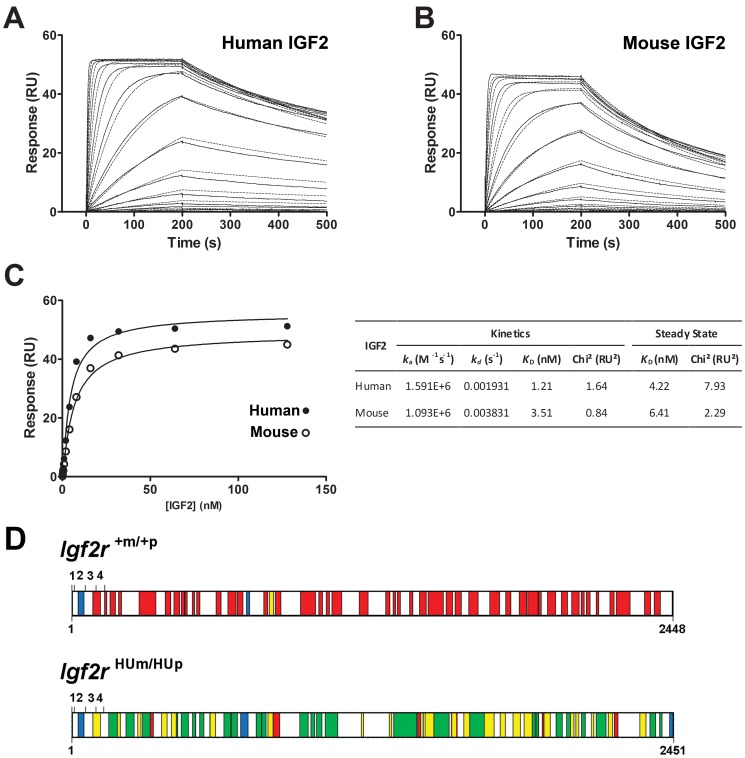
Figure 7. Surface Plasmon resonance binding kinetics of IGF2 binding to human IGF2R, and mass spectrometry (LCMS) of immune-precipitated liver IGF2R from control Igf2r+m/+p and homozygote transmission of Igf2r+HUm/HUp.
Immobilised human IGF2R on a biosensor chip were bound to either recombinant human (A) or mouse (B) IGF2 at increasing concentrations, and real time kinetics fitted to a 1∶1 binding model. (C) steady state binding kinetics were also calculated for both interactions and tabulated. Mouse IGF2 has slightly lower KD (nM). (D) peptides identified by mass spectrometry in samples of immuno-precipitated Igf2r+m/+p (WT) and Igf2rHUm/HUp. In both samples, red boxes depict unique peptides matching mouse IGF2R protein sequence, green boxes depict unique peptides matching human IGF2R protein sequence, and yellow boxes depict areas of overlap between mouse and human peptides. Blue boxes depict peptides common to both sequences. Exons 1–4 are represented by upper numbers, lower numbers represent amino acid residue number. Both mouse and human peptides were detectable in suggesting trans-splicing and generation of mouse and humanised proteins from the humanised alleles.