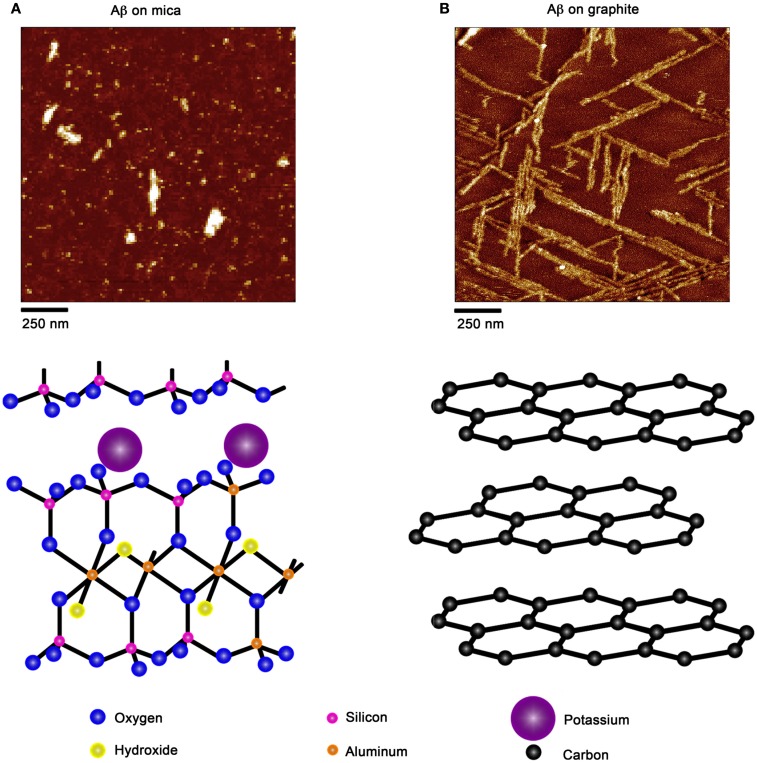
Figure 2.
Aβ aggregation is modulated by the presence of chemically distinct solid surfaces. (A) On highly ordered pyrolytic graphite, Aβ aggregates into extended nanoribbons that are epitaxially ordered on the surface. The distinct orientation of Aβ aggregates on graphite is attributed to the optimization of the contact between the peptide and underlying hydrophobic carbon lattice. (B) On a negatively charged, hydrophilic mica surface, Aβ forms discrete oligomers that maintained some lateral mobility along the plane of the surface. These oligomers could organize into elongated protofibrillar structures. Schematic representations of the structure of each surface (graphite and mica) are provided under each image.