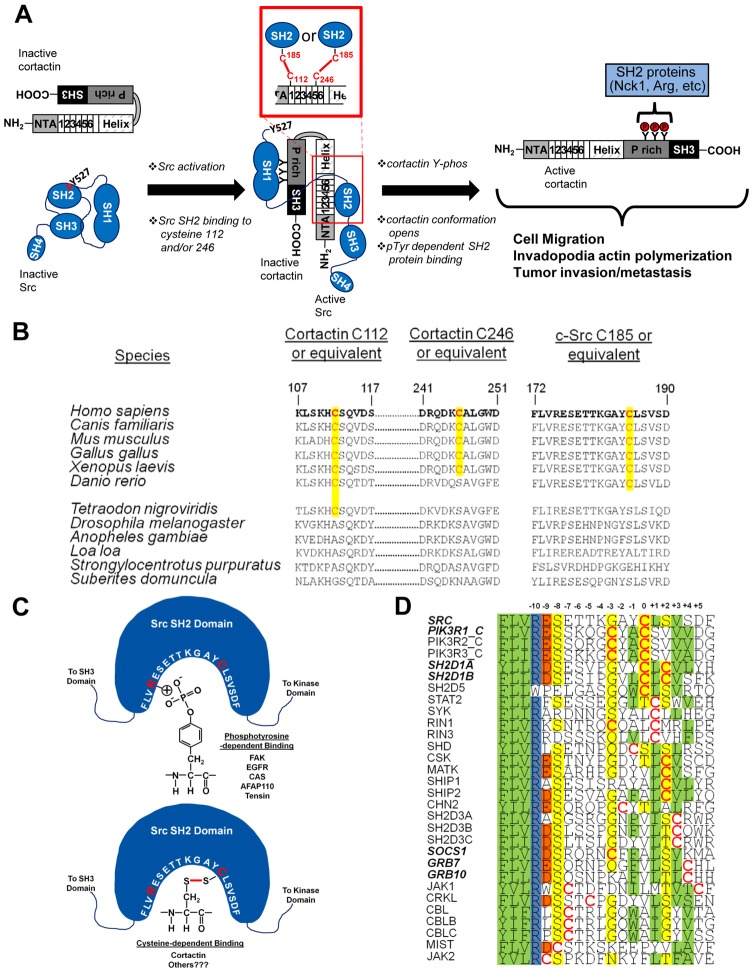
Fig. 7.
Model of cysteine-mediated interactions in cortactin regulation. (A) Model of cysteine-based cortactin activation and phosphorylation by Src. (B) Phylogenetic co-conservation of cortactin C112/246 and Src C185. Conserved cysteines are in red and the homologous positions highlighted in yellow. (C) Cartoon representation of Src SH2 binding to phosphotyrosine and cysteine residues. Src amino acids 172–191 within the SH2 domain binding pocket are in white; interacting arginine 175 and cysteine 185 residues are in red. Cystine bonding is indicated as a red line. Phosphotyrosine and cystine binding ligands are listed. (D) Alignment of cysteine-containing SH2 domains. Domains known to bind ligands in a phosphotyrosine-independent manner are in bold italics. Cysteine residues are in red. Shading: green; hydrophobic, blue; positively charged, red; negatively charged, yellow; polar.