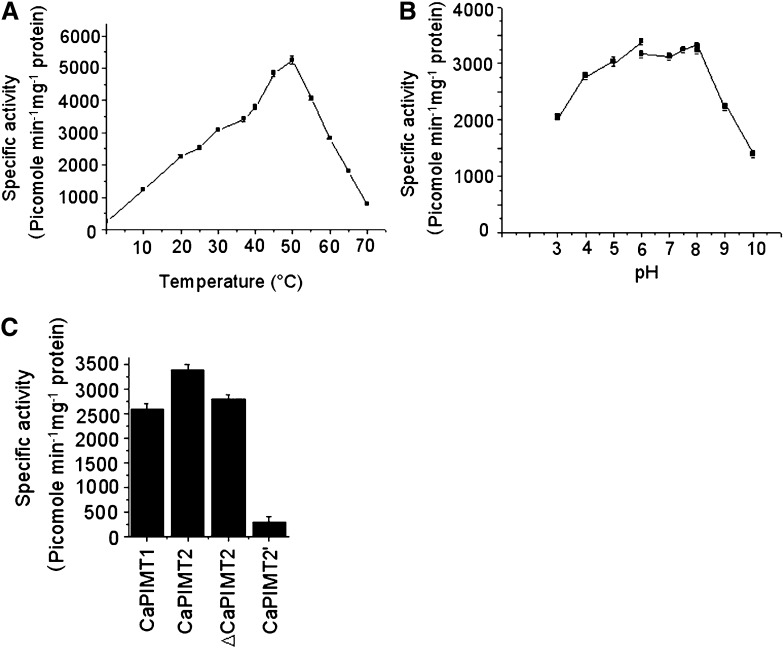
Figure 4.
Biochemical characterization of the CaPIMT2 enzyme. A, Effect of temperature on the activity of purified recombinant CaPIMT2 enzyme. The specific activity was measured at different temperatures ranging from 0°C to 70°C. B, Effect of pH on the activity of purified recombinant CaPIMT2. The specific activity was measured at 37°C using different buffers (pH 3–6, citrate phosphate buffer; pH 6–8, sodium phosphate buffer; pH 8–10, 2-amino-2-methyl-1,3-propanediol buffer). C, Comparison of enzyme activity among CaPIMT1, CaPIMT2, ΔCaPIMT2 (CaPIMT2 lacking the 56-amino acid N terminus), and CaPIMT2′. Approximately 5 μg of purified protein was assayed in each case. Error bars in all graphs indicate se from three independent experiments.