Fig. 4.
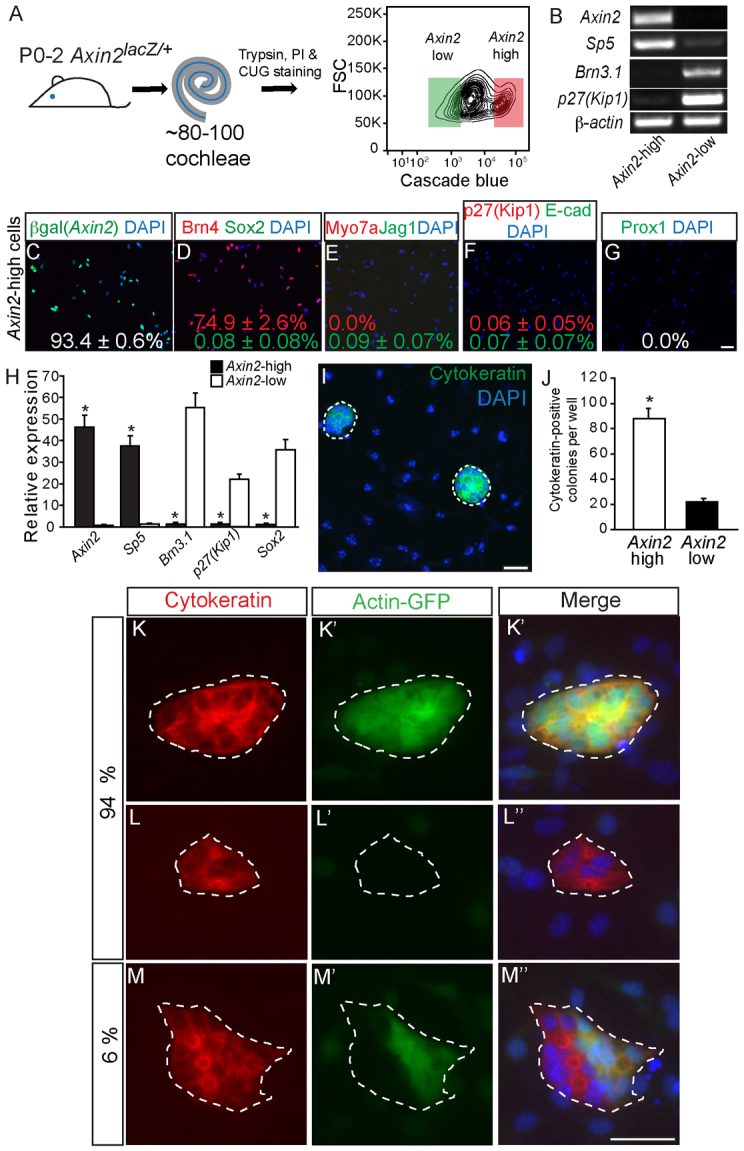
Purified Axin2hi cells display robust proliferative capacity. (A) P0-P2 Axin2lacZ/+ cochleae were dissociated and labeled with propidium iodide (PI) and CUG to allow for isolation of PI-negative (viable), CUG-positive cells. The flow cytometry plot depicts gates used for isolation of the Axin2hi (red) and Axin2lo (green) cells. (B-H) Isolated CUG-positive cells robustly expressed the Wnt target genes Axin2 and Sp5, and not the hair cell marker Brn3.1 or the supporting cell markers p27Kip1 and Sox2 (P<0.01 for all). Experiments were carried out in triplicate. Immunostaining of CUG-positive cells shows 93.4±0.6% β-gal-positive, 74.9±2.6% Brn4-positive, 0.0% myosin 7a-positive hair cells (0.0%) and rare (<0.1%) (Sox2-, jagged 1-, p27Kip1-, E-cadherin or Prox1-positive) supporting cells. (I,J) After 10 days in vitro, Axin2hi cells formed cytokeratin-positive colonies more frequently than Axin2lo cells (88.2±19.4 versus 22.0±6.2, P<0.0001, n=5). (K-M″) Axin2hi cells isolated from both Actin-GFP-positive Axin2lacZ/+ cochleae and Actin-GFP-negative Axin2lacZ/+ cochleae were mixed 1:1 and cultured. After 10 days, 94% of cytokeratin-positive colonies were monochromatic, suggesting Axin2hi colonies were highly clonal (n=3). Scale bars: 25 μm. Data are mean±s.d. Asterisks indicate statistical significance.
