Fig. 1.
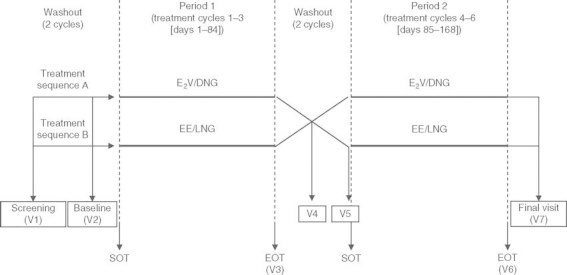
Design of the crossover study. E 2V/DNG = estradiol valerate/dienogest; = ethinylestradiol/levonorgestrel; EOT = end of treatment; SOT = start of treatment (first day of bleeding); V1 = screening visit; V2 = baseline (days 15–21 of the cycle); V3 = end of treatment period 1 (end of treatment cycle 3).[E2V/DNG = treatment days 74–80; EE/LNG = treatment days 71–77]; V4 = end of washout cycle 1 (days 15–21 of the cycle); V5 = end of washout cycle 2 (days 15–21 of the cycle)/baseline for treatment period 2; V6 = end of treatment period 2 (end of treatment cycle 6).[E2V/DNG = treatment days 158–164; EE/LNG = treatment days 155–161]; V7 = up to 2 weeks after the end of treatment, but at least 2 days after the end of the withdrawal bleeding that followed treatment cycle 6.
