Fig. 1.
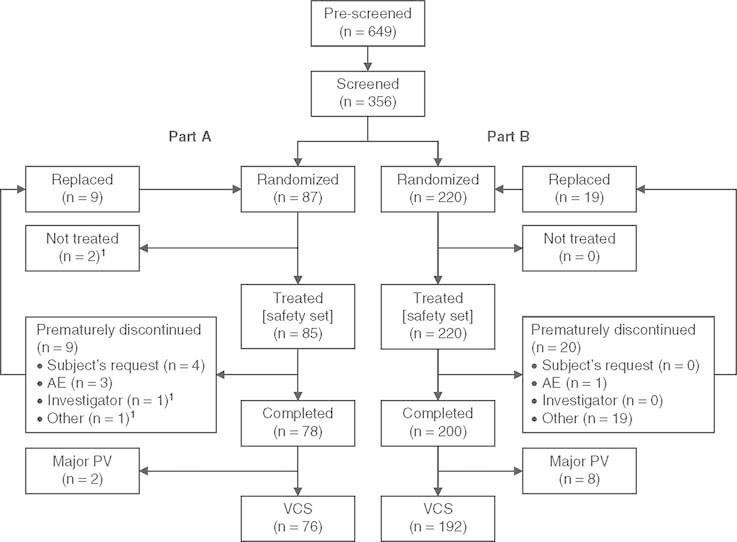
Subject randomization and analysis populations. Two randomized subjects in part A were not treated and were withdrawn from the study for the following reported reasons: ‘high blood pressure pre-dose’ and ‘poor vein conditions’. AE = adverse event; PV = protocol violation; VCS = valid cases set for pharmacokinetic and pharmacodynamic analyses.
