Abstract
Objectives
To describe the hospitalisation patterns in children with intellectual disability (ID) and/or autism spectrum disorder (ASD) after the first year of life and compare with those unaffected.
Design
Prospective cohort study using data linkage between health, ID and hospitalisation population-based datasets.
Setting
Western Australia.
Participants
416 611 individuals born between 1983 and 1999 involving 1 027 962 hospital admission records. Five case categories were defined (mild/moderate ID, severe ID, biomedically caused ID, ASD with ID and ASD without ID) and compared with the remainder of children and young people.
Primary and secondary outcome measures
Time to event analysis was used to compare time hospitalisation and rate of hospitalisation between the different case-groups by estimating HR, accounting for birth year and preterm birth status.
Results
ID and/or ASD were found to be associated with an increased risk of hospitalisation compared with the remainder of the population. The increase in risk was highest in those with severe ID and no ASD (HR=10.33, 95% CI 8.66 to 12.31). For those with ID of known biomedical cause or mild ID of unknown cause, the risk of hospitalisation was lower (HR=7.36, 95% CI 6.73 to 8.07 and HR=3.08, 95% CI 2.78 to 3.40, respectively). Those with ASDs had slightly increased risk (HR=2.82, 95% CI 2.26 to 3.50 for those with ID and HR=2.09, 95% CI 1.85 to 2.36 for those without ID).
Conclusions
Children with an ID or ASD experience an increased risk of hospitalisation after the first year of life which varied from 2 to 10 times that of the rest of the population. Findings can inform service planning or resource allocation for these children with special needs.
Keywords: EPIDEMIOLOGY, PUBLIC HEALTH
Article summary.
Article focus
Children with an intellectual disability (ID) and/or autism often experience co-occurring morbidities.
The rate of hospital admissions experienced by these children compared with the rest of the population has not been detailed previously.
Key messages
The risk of hospitalisation for children with ID or autism is up to 10 times that of those unaffected.
The risk is greatest for those with severe ID without the presence of autism.
Preterm birth was a risk factor for later hospitalisation independent of the year of birth or the presence of ID or autism.
Strengths and limitations of this study
The strength of our study has been the ability to use population-based data on children's hospitalisations and link these to sources providing information on the presence of ID or autism spectrum disorders.
One weakness in the study included the exclusion from our modelling of hospitalisation history in the first year of life.
Introduction
Intellectual disability (ID) affects 143/10 000 children1 and is associated with a range of comorbid health conditions.2–4 It is heterogeneous,5 and clustering of some medical conditions may be associated with particular disorders such as Down syndrome6 or Prader-Willi syndrome.7 While epilepsy and sensory impairments often occur in association with specific syndromes or more severe cognitive impairment, conditions such as fractures or obesity may develop as secondary to medication use, nutritional deficiency or lack of mobility.2 Consequently, children with ID may face greater health challenges than typically developing children and use healthcare systems more frequently.8 9 Mental health problems are also common in people with ID.10 For instance, in a Canadian adolescent and adult population with ID, a high proportion of hospitalisations was attributed to the presence of psychiatric conditions.11
Autism spectrum disorders (ASD) are neurodevelopmental disorders characterised by difficulties in communication and social interaction and associated with repetitive or unusual behaviours.12 ASD was reported to affect 30 and 60/10 000 children in 2003.13 However, a more recent report from the US Autism and Developmental Disabilities Monitoring Network suggests a higher prevalence of 113/10 000.14 Moreover, nearly two-thirds of children with ASDs also have ID.15 Therefore, it is not surprising that children with ASD also often experience medical conditions such as epilepsy, bowel dysfunction and autoimmune disorders16 and that their burden of hospitalisation is considered to be high.17
Differences in healthcare policies and pathways of care may impact on hospitalisation rates and service use for children with developmental disorders over time.8 18 For example, a recent Canadian study using administrative data19 found that those with intellectual or developmental disabilities were more likely than those without to use emergency department services. Families with children affected by ID and/or ASDs may have particular difficulty in accessing primary and specialist healthcare services, leading to emergency department presentations and hospitalisation rates greater than those not affected by these disorders. A further Canadian study20 found that admissions to an emergency department for people with ID were more likely to be for a psychobehavioural rather than a medical reason. However, there are less population-based data describing the pattern of hospitalisation among children with ID or ASD. This study uses data linkage between health and disability datasets to investigate temporal trends in hospitalisation risk for children with ID and children with ASD in Western Australia (WA) born 1983–1999. The aim was to compare hospitalisation rates between children with ID and/or ASD and children without these diagnoses, taking into account the type of ID, birth cohort and preterm birth.
Methods
Cases of ID were ascertained from the Intellectual Disability Exploring Answers (IDEA) database,21 a population-based register of individuals in WA with ID with or without an ASD. The IDEA database receives information from the Disability Services Commission (DSC) and from the Department of Education on individuals accessing services or educational support for an ID in WA. The inclusion criteria for the IDEA database are an indication of developmental delay before 18 years of age, a full IQ score below 70, and significant deficits in adaptive behaviour. Cases are categorised into mild ID (IQ between 55 and 69), moderate ID (IQ 40–54) and severe ID (IQ <40), but for the purposes of this study, the mild and moderate ID groups were combined.
As detailed previously,15 individuals with a diagnosis of an ASD (which includes autism, Asperger syndrome or Pervasive Developmental Disorder not otherwise specified) were again identified for this study from three sources: DSC, the Western Australian Register for ASDs (a prospective data collection system for diagnostic information for cases diagnosed since 1999) and/or from a case group born 1983–1995 and diagnosed by 1999 as identified by a developmental paediatrician through case-note review. Using record linkage to the IDEA database, individuals identified with an ASD were subdivided into those with or without an additional ID. Those for whom an ID status could not be determined were grouped with those with ID, so that the ASD without ID group contained only those definitively known not to have an ID. Individuals were further classified according to whether there was a known biomedical cause for the ID (such as a congenital or genetic condition, eg, Down syndrome) or otherwise. This categorisation is based on the information in the IDEA Database21 where assignment of diagnosis was made by the attending clinician according to an AAMR classification system. In this study, we followed a similar protocol to that used in our own15 22 and previous published research.23 Biomedical diagnoses include genetic conditions (chromosomal and Mendelian), recognised teratogenic effects such as congenital infections and birth defects, neonatal and postneonatal infections, trauma and other events (eg, neoplasm). However, we excluded diagnostic categories (eg, preterm birth), which are associated with but are not necessarily a sufficient cause of ID, and those where a genetic diagnosis might be suspected but not clinically or genetically confirmed.
Therefore, five case categories were defined (mild/moderate ID, severe ID, biomedically caused ID, ASD with ID and ASD without ID) and compared with the remainder of children and young people born in WA between 1983 and 1999 who, at the time of case extraction, were unaffected by ID or ASD.
Information on hospitalisations from 1 January 1983 to 31 December 2004 was obtained from the WA Hospital Morbidity Data System, which is a statutory collection of data relating to all inpatient episodes to public, private and freestanding day hospitals in WA since 1970.24 It contains ICD-coded diagnosis and procedure information. WA also has a statutory collection of pregnancy, birth and maternal data, recorded at the time of birth, known as the Midwives Notification System.24 Linkage of all birth records 1983–1999 to hospital and death data were undertaken independently at the WA Data Linkage Branch, where these datasets and others are linked regularly for the purposes of approved research projects.24 A deidentified dataset was provided for this analysis in which all individuals were followed from birth through to either death or the censoring date of 31 December 2004. Preterm birth was defined as being born at less than 37 weeks gestation with gestational age obtained from the Midwives Notification System.
The analysis was restricted to hospitalisations occurring after the first year of life, thereby excluding individuals who did not survive to this age. The focus of this paper was on hospitalisations occurring in childhood and adolescence, an area lacking in quantitative, population-based research, rather than on the hospitalisations occurring perinatally or in infancy, where our group has already quantified the increased burden of hospital care in children with ID (particularly biomedically caused ID).8 Hospitalisations occurring on the same day as another recorded hospitalisation, as well as those occurring as part of a nested transfer (the patient moves to another hospital during a stay in hospital) or a transfer sequence (the patient moves between hospitals successively without returning home), were considered as a single event.
While we refer to case and comparison groups throughout, this is a cohort study where the different case groups can be thought of as different ‘exposures’. Thus, time-to-event analysis (which allows for varying individual time at risk) was used to compare time to hospitalisation and rate of hospitalisation between the groups, accounting for birth year and preterm birth status. Hospitalisation rates were represented by incidence rates (IR) in terms of number of admissions per person per year. Group status was used as a categorical predictor of time to hospitalisation, producing Nelson-Aalen cumulative hazard estimates (which in this case represent the expected number of hospitalisations experienced by a particular age). HRs from the Cox proportional hazards model were also used to investigate differences in risk of hospitalisation for the different case groups, with robust SEs estimated to account for the multiple hospitalisations per individual. The effect of birth year was considered by stratifying the year of birth into four eras being 1983–1985, 1986–1989, 1990–1993 and 1994–1999, such that each group has an equal number of records, using STATA's egen cut command. In more specific modelling, birth year was included as an indicator variable for each individual year from 1983 to 1999 rather than banded. Ethical approval for this study was granted by the UWA Human Research Ethics Committee.
Results
A dataset of 1 027 962 records representing the time at risk of hospitalisation for 416 611 children (table 1) and young people born in WA from 1983 to 1999 was created. The total time at risk of hospitalisation in the cohort was 5 146 927.5 person-years, while 3818.6 person-years were spent in hospital (when not on the risk set for hospitalisation). Individual time at risk varied from 5 (for those born in 1999) to 21 years (for those born in 1983). The dataset contained 611 816 admissions to hospital with a median of one hospitalisation per subject, giving an IR of 0.12 admissions per person per year and median time to hospitalisation of 5.09 years (IQR 2.26–13.65 years). By the age of 10 years, 67.7% (95% CI 67.6% to 67.8%) of individuals had been hospitalised at least once, increasing to 85% (95% CI 84.9% to 85.1%) by the age of 18 years. On average, a child of 10 years in this cohort had been hospitalised 1.13 times (cumulative hazard) and a young person of 18 years, 1.90 times.
Table 1.
Distribution of individuals in different case groups
| Case-comparison group status | Frequency | Per cent | Cumulative |
|---|---|---|---|
| No ASD no ID | 409454 | 98.28 | 98.28 |
| Mild/mod ID no ASD | 4667 | 1.12 | 99.4 |
| Severe ID no ASD | 293 | 0.07 | 99.47 |
| ASD & ID | 767 | 0.18 | 99.66 |
| ASD no ID | 475 | 0.11 | 99.77 |
| Biomedical ID | 955 | 0.23 | 100 |
| Total | 416611 | 100 |
ASD,autism spectrum disorder; ID,intellectual disability.
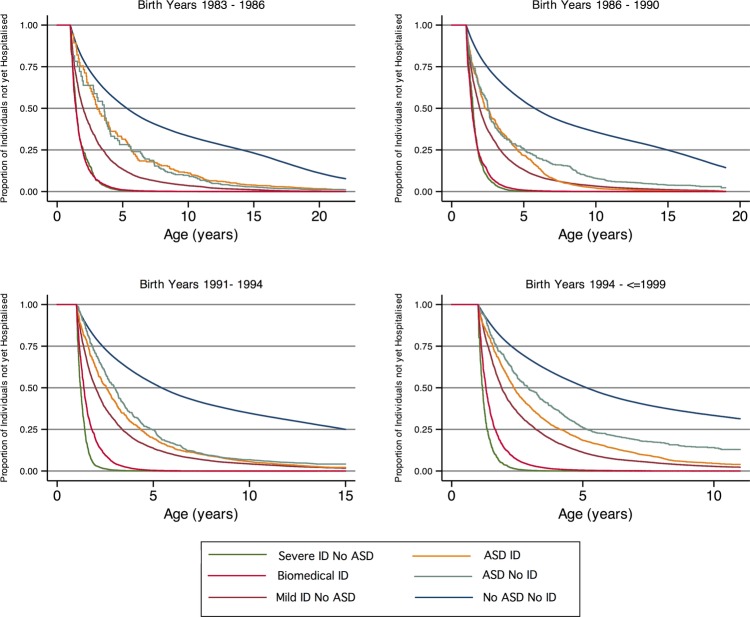
The highest rate of hospitalisation was observed for cases with severe ID but without an ASD (1.16 hospitalisations/person-year, median time to hospitalisation 1.27 years) followed by cases with a biomedical cause of ID (0.84 hospitalisations/person year, median age of 1.37 years at first hospitalisation), mild or moderate ID without ASD (IR 0.34, median age 1.95 years) and ASDs with ID (IR 0.33, median age of 2.43 years at first hospitalisation). Those children with ASD and no ID had the lowest rate of hospitalisation of the case groups (IR 0.24, median age of 2.89 years at first hospitalisation); however, their rate was still higher than that of the general population (IR 0.11, median age of 5.37 years at first hospitalisation). The highest rates of hospitalisation over time were experienced by those with severe ID and those with ID with a known biomedical cause, with both groups experiencing five or more hospitalisations by 5 years of age (figure 1). The similarity of curves between those with mild/moderate ID and ASD with ID can also be seen. All case groups experienced a higher estimated risk of hospitalisations than the unaffected individuals (table 2).
Figure 1.
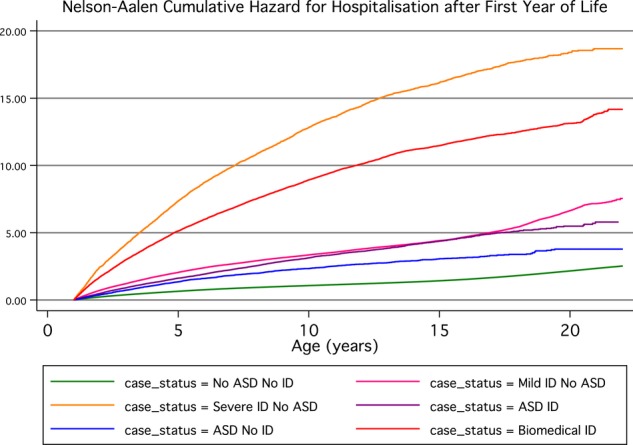
Nelson-Aalen cumulative hazard for hospitalisation (number of hospitalisations expected by age shown on x-axis).
Table 2.
Cox proportional HRs for hospitalisation by case group
| Case group | HR | Robust SE | p Value* | 95% CI |
||
|---|---|---|---|---|---|---|
| No ASD no ID | 1.0 | – | ||||
| Mild/mod ID no ASD | 3.1 | 0.16 | <0.001 | 2.8 | 3.4 | |
| Severe ID no ASD | 10.3 | 0.93 | <0.001 | 8.7 | 12.3 | |
| ASD & ID | 2.8 | 0.31 | <0.001 | 2.3 | 3.5 | |
| ASD no ID | 2.1 | 0.13 | <0.001 | 1.8 | 2.4 | |
| Biomedical ID | 7.4 | 0.34 | <0.001 | 6.7 | 8.1 | |
*Wald test.
ASD,autism spectrum disorder; ID,intellectual disability.
Figure 2 shows the time to hospitalisation curves by case status for the four birth cohorts. The curves for the severe ID and the biomedically caused ID groups separate increasingly with advancing age and show that the difference between these two groups has amplified over time. Table 3 shows the differences in rate estimates for the different birth year bands over the different case status groups, compared with all other groups. This shows that there is an increase in the difference in hospitalisation rate over time in the severe ID (no ASD) groups and the biomedical ID groups that is much greater than the rate differences of the other groups, and that the severe ID group's risk increases more steeply than that of the biomedical ID group. To measure this more precisely, the interaction effects between case status and birth year were included in the Cox proportional hazards modelling and demonstrated that a significantly greater proportion of the variability in time to hospitalisation was explained by the interaction model than by the main effects model (p < 0.001).
Figure 2.
Kaplan-Meier curves showing the proportion of individuals in each case group who have not been hospitalised by age, for each birth cohort.
Table 3.
Rate estimate comparison (using margins, dydx) for birth year band trend over the different case-status groups
| Birth year band | Case-status | Rate estimate difference | 95% CI | p Value | ||
|---|---|---|---|---|---|---|
| 1983–1986 | Affected | Baseline for affected status rate estimate | ||||
| No ASD no ID | Baseline for no ASD no ID rate estimate | |||||
| 1986–1990 | Affected | 0.573 | 0.445 | 0.701 | <0.001 | |
| No ASD no ID | −0.046 | −0.053 | −0.039 | <0.001 | ||
| 1990–1994 | Affected | 0.056 | 0.010 | 0.102 | 0.017 | |
| No ASD no ID | −0.266 | −0.811 | 0.278 | 0.338 | ||
| 1994–1999 | Affected | 0.075 | 0.028 | 0.122 | 0.002 | |
| No ASD no ID | 0.263 | −0.388 | 0.913 | 0.429 | ||
| 1983–1986 | All other groups | Baseline for all other groups rate estimate | ||||
| Mild ID no ASD | Baseline for mild ID no ASD rate estimate | |||||
| 1986–1990 | All other groups | −0.014 | −0.023 | −0.005 | 0.001 | |
| Mild ID no ASD | −0.210 | −0.328 | −0.091 | 0.001 | ||
| 1990–1994 | All other groups | 0.042 | 0.032 | 0.052 | <0.001 | |
| Mild ID no ASD | −0.332 | −0.452 | −0.212 | <0.001 | ||
| 1994–1999 | All other groups | 0.081 | 0.070 | 0.091 | <0.001 | |
| Mild ID no ASD | 0.084 | −0.056 | 0.224 | 0.239 | ||
| 1983–1986 | All other groups | Baseline for all other groups rate estimate | ||||
| Severe ID no ASD | Baseline for severe ID no ASD rate estimate | |||||
| 1986–1990 | All other groups | −0.035 | −0.043 | −0.026 | <0.001 | |
| Severe ID no ASD | 3.570 | 2.905 | 4.236 | <0.001 | ||
| 1990–1994 | All other groups | −0.001 | −0.010 | 0.009 | 0.841 | |
| Severe ID no ASD | 7.901 | 7.042 | 8.760 | <0.001 | ||
| 1994–1999 | All other groups | 0.040 | 0.030 | 0.050 | <0.001 | |
| Severe ID no ASD | 9.399 | 8.312 | 10.487 | <0.001 | ||
| 1983–1986 | All other groups | Baseline for all other groups rate estimate | ||||
| ASD & ID | Baseline for ASD & ID rate estimate | |||||
| 1986–1990 | All other groups | −0.021 | −0.030 | −0.012 | <0.001 | |
| ASD & ID | 1.488 | 1.115 | 1.862 | <0.001 | ||
| 1990–1994 | All other groups | 0.046 | 0.034 | 0.057 | <0.001 | |
| ASD & ID | 0.855 | 0.467 | 1.242 | <0.001 | ||
| 1994–1999 | All other groups | 0.079 | 0.068 | 0.090 | <0.001 | |
| ASD & ID | 0.835 | 0.502 | 1.168 | <0.001 | ||
| 1983–1986 | All other groups | Baseline for all other groups rate estimate | ||||
| ASD no ID | Baseline for ASD & no ID rate estimate | |||||
| 1986–1990 | All other groups | −0.016 | −0.025 | −0.008 | <0.001 | |
| ASD no ID | 0.104 | −0.321 | 0.530 | 0.631 | ||
| 1990–1994 | All other groups | 0.048 | 0.036 | 0.059 | <0.001 | |
| ASD no ID | 0.493 | 0.057 | 0.929 | 0.027 | ||
| 1994–1999 | All other groups | 0.082 | 0.071 | 0.093 | <0.001 | |
| ASD no ID | −0.064 | −0.463 | 0.335 | 0.754 | ||
| 1983–1986 | All other groups | Baseline for all other groups rate estimate | ||||
| Biomedical ID | Baseline for biomedical ID rate estimate | |||||
| 1986–1990 | All other groups | −0.031 | −0.039 | −0.023 | <0.001 | |
| Biomedical ID | 1.439 | 1.100 | 1.777 | <0.001 | ||
| 1990–1994 | All other groups | −0.005 | −0.015 | 0.005 | 0.340 | |
| Biomedical ID | 5.307 | 4.828 | 5.786 | <0.001 | ||
| 1994–1999 | All other groups | 0.055 | 0.044 | 0.065 | <0.001 | |
| Biomedical ID | 2.731 | 2.322 | 3.140 | <0.001 | ||
ASD, autism spectrum disorder; ID, intellectual disability.
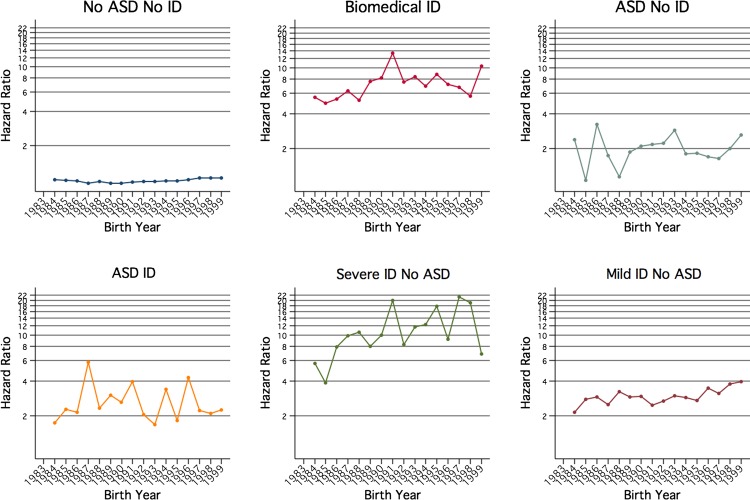
Figure 3 shows the HRs over the birth year from this interaction model in all six groups. These HRs and their CIs are also shown in table 4. The great increase in risk for all groups compared with the general population is clear, as is the change in hospitalisation relative risk over time both within each group and the differing patterns between the groups. In all groups, an increase in the risk of hospitalisation was associated with a more recent birth year; however, a small but steadily upward trend was only observed in the general population and the mild/moderate ID groups. The risk of hospitalisation per birth year fluctuated for those with ASDs (with or without an ID) and for those with severe ID (though from a higher rate).
Figure 3.
HRs from multivariate interaction model by birth year and case group status.
Table 4.
HRs, 95% CIs and p values compared with individuals without ASD or ID born in 1993 as shown in figure 3
| Case status | Birth year | HR | 95% CI |
p Value (compared with baseline) | |
|---|---|---|---|---|---|
| No ASD or ID | 1983 | Baseline | |||
| 1984 | 0.997 | 0.985 | 1.009 | 0.587 | |
| 1985 | 0.994 | 0.982 | 1.007 | 0.352 | |
| 1986 | 0.974 | 0.962 | 0.987 | <0.001 | |
| 1987 | 0.930 | 0.918 | 0.943 | <0.001 | |
| 1988 | 0.960 | 0.947 | 0.973 | <0.001 | |
| 1989 | 0.934 | 0.921 | 0.947 | <0.001 | |
| 1990 | 0.929 | 0.915 | 0.942 | <0.001 | |
| 1991 | 0.946 | 0.932 | 0.960 | <0.001 | |
| 1992 | 0.960 | 0.946 | 0.974 | <0.001 | |
| 1993 | 0.968 | 0.954 | 0.982 | <0.001 | |
| 1994 | 0.972 | 0.958 | 0.987 | <0.001 | |
| 1995 | 0.975 | 0.960 | 0.990 | 0.001 | |
| 1996 | 0.999 | 0.984 | 1.015 | 0.943 | |
| 1997 | 1.036 | 1.019 | 1.052 | <0.001 | |
| 1998 | 1.041 | 1.024 | 1.058 | <0.001 | |
| 1999 | 1.031 | 1.014 | 1.049 | <0.001 | |
| Mild ID no ASD | 1983 | 4.626 | 4.418 | 4.844 | <0.001 |
| 1984 | 2.192 | 2.061 | 2.332 | <0.001 | |
| 1985 | 2.852 | 2.704 | 3.009 | <0.001 | |
| 1986 | 2.958 | 2.811 | 3.112 | <0.001 | |
| 1987 | 2.558 | 2.436 | 2.686 | <0.001 | |
| 1988 | 3.328 | 3.175 | 3.488 | <0.001 | |
| 1989 | 3.014 | 2.869 | 3.166 | <0.001 | |
| 1990 | 3.019 | 2.875 | 3.170 | <0.001 | |
| 1991 | 2.539 | 2.408 | 2.678 | <0.001 | |
| 1992 | 2.736 | 2.601 | 2.879 | <0.001 | |
| 1993 | 3.079 | 2.904 | 3.266 | <0.001 | |
| 1994 | 3.017 | 2.848 | 3.197 | <0.001 | |
| 1995 | 2.821 | 2.632 | 3.024 | <0.001 | |
| 1996 | 3.662 | 3.421 | 3.920 | <0.001 | |
| 1997 | 3.254 | 2.960 | 3.577 | <0.001 | |
| 1998 | 3.896 | 3.498 | 4.339 | <0.001 | |
| 1999 | 4.001 | 3.549 | 4.510 | <0.001 | |
| Severe ID no ASD | 1983 | 6.813 | 6.046 | 7.676 | <0.001 |
| 1984 | 5.783 | 5.052 | 6.620 | <0.001 | |
| 1985 | 4.619 | 4.014 | 5.314 | <0.001 | |
| 1986 | 8.095 | 7.252 | 9.038 | <0.001 | |
| 1987 | 10.362 | 9.257 | 11.599 | <0.001 | |
| 1988 | 11.129 | 10.030 | 12.348 | <0.001 | |
| 1989 | 8.089 | 7.245 | 9.033 | <0.001 | |
| 1990 | 11.263 | 10.049 | 12.625 | <0.001 | |
| 1991 | 20.315 | 18.789 | 21.965 | <0.001 | |
| 1992 | 8.552 | 7.491 | 9.763 | <0.001 | |
| 1993 | 11.824 | 10.050 | 13.909 | <0.001 | |
| 1994 | 12.425 | 10.556 | 14.626 | <0.001 | |
| 1995 | 17.095 | 14.755 | 19.806 | <0.001 | |
| 1996 | 9.998 | 8.007 | 12.485 | <0.001 | |
| 1997 | 20.663 | 17.483 | 24.422 | <0.001 | |
| 1998 | 20.499 | 18.260 | 23.013 | <0.001 | |
| 1999 | 7.245 | 5.612 | 9.353 | <0.001 | |
| ASD & ID | 1983 | 1.352 | 0.945 | 1.934 | 0.099 |
| 1984 | 1.743 | 1.313 | 2.313 | <0.001 | |
| 1985 | 2.206 | 1.745 | 2.789 | <0.001 | |
| 1986 | 2.081 | 1.664 | 2.602 | <0.001 | |
| 1987 | 5.996 | 5.353 | 6.715 | <0.001 | |
| 1988 | 2.246 | 1.838 | 2.744 | <0.001 | |
| 1989 | 3.081 | 2.665 | 3.563 | <0.001 | |
| 1990 | 2.642 | 2.227 | 3.134 | <0.001 | |
| 1991 | 4.074 | 3.579 | 4.638 | <0.001 | |
| 1992 | 2.039 | 1.696 | 2.450 | <0.001 | |
| 1993 | 1.762 | 1.428 | 2.175 | <0.001 | |
| 1994 | 3.448 | 3.021 | 3.936 | <0.001 | |
| 1995 | 1.821 | 1.516 | 2.186 | <0.001 | |
| 1996 | 4.367 | 3.872 | 4.925 | <0.001 | |
| 1997 | 2.225 | 1.927 | 2.568 | <0.001 | |
| 1998 | 2.102 | 1.800 | 2.454 | <0.001 | |
| 1999 | 2.264 | 1.936 | 2.647 | <0.001 | |
| ASD no ID | 1983 | 2.048 | 1.508 | 2.782 | <0.001 |
| 1984 | 2.321 | 1.773 | 3.038 | <0.001 | |
| 1985 | 1.095 | 0.671 | 1.787 | 0.718 | |
| 1986 | 3.387 | 2.661 | 4.312 | <0.001 | |
| 1987 | 1.720 | 1.338 | 2.211 | <0.001 | |
| 1988 | 1.104 | 0.712 | 1.712 | 0.657 | |
| 1989 | 1.936 | 1.527 | 2.456 | <0.001 | |
| 1990 | 2.088 | 1.690 | 2.580 | <0.001 | |
| 1991 | 2.139 | 1.777 | 2.575 | <0.001 | |
| 1992 | 2.207 | 1.843 | 2.645 | <0.001 | |
| 1993 | 2.928 | 2.471 | 3.469 | <0.001 | |
| 1994 | 1.819 | 1.477 | 2.239 | <0.001 | |
| 1995 | 1.874 | 1.524 | 2.305 | <0.001 | |
| 1996 | 1.691 | 1.384 | 2.066 | <0.001 | |
| 1997 | 1.638 | 1.293 | 2.074 | <0.001 | |
| 1998 | 2.080 | 1.562 | 2.768 | <0.001 | |
| 1999 | 2.728 | 2.001 | 3.720 | <0.001 | |
| Biomedical ID | 1983 | 4.812 | 4.443 | 5.211 | <0.001 |
| 1984 | 5.748 | 5.328 | 6.200 | <0.001 | |
| 1985 | 5.012 | 4.615 | 5.444 | <0.001 | |
| 1986 | 5.480 | 5.041 | 5.958 | <0.001 | |
| 1987 | 6.311 | 5.832 | 6.829 | <0.001 | |
| 1988 | 5.369 | 4.900 | 5.883 | <0.001 | |
| 1989 | 7.738 | 7.095 | 8.438 | <0.001 | |
| 1990 | 8.700 | 8.089 | 9.358 | <0.001 | |
| 1991 | 13.724 | 12.984 | 14.505 | <0.001 | |
| 1992 | 7.796 | 7.161 | 8.488 | <0.001 | |
| 1993 | 8.786 | 8.127 | 9.497 | <0.001 | |
| 1994 | 7.086 | 6.355 | 7.902 | <0.001 | |
| 1995 | 8.710 | 8.017 | 9.463 | <0.001 | |
| 1996 | 7.535 | 6.798 | 8.353 | <0.001 | |
| 1997 | 7.182 | 6.403 | 8.057 | <0.001 | |
| 1998 | 6.102 | 5.370 | 6.935 | <0.001 | |
| 1999 | 10.575 | 9.584 | 11.667 | <0.001 | |
ASD, autism spectrum disorder; ID, intellectual disability.
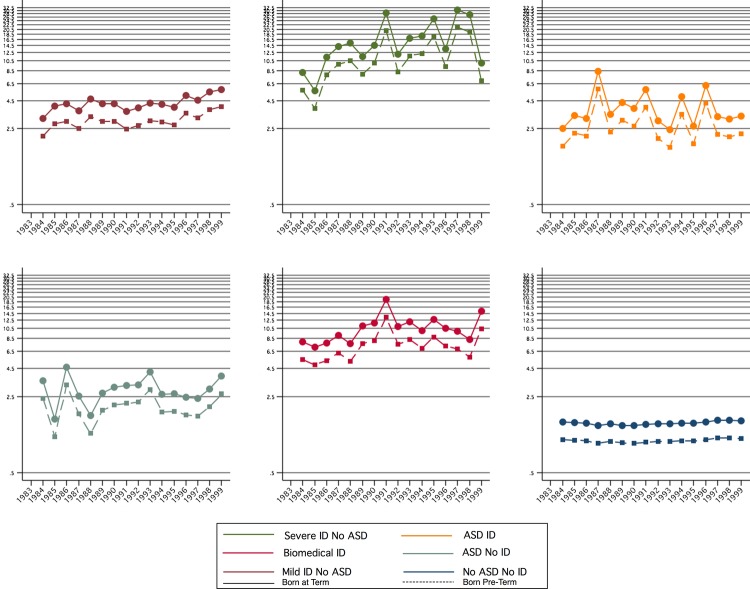
There were 30 850 births before 37 weeks gestation (7.42% of all births in the cohort) who survived the first year of life. Univariate comparison of time to hospitalisation for those born preterm versus full term suggests that those born preterm are admitted to hospital more frequently (IR 0.17 per person-year for preterm, 0.11 for full term), and earlier (median time to hospitalisation 3.01 years for preterm, 5.37 years for full-term) than those born full term. Including birth year and case status factors in the Cox proportional hazards model showed that the HR for preterm birth was 1.45 (95% CI 1.39 to 1.51, p<0.001), indicating the independent effect of preterm birth on hospitalisations for each case group (figure 4).
Figure 4.
HR for hospitalisation risk associated with birth year, preterm birth and case group status from stratified model.
Discussion
This study quantified the rate of hospitalisations for children with an ID and/or ASD compared with the general population. Children diagnosed with ID and/or ASD experienced more hospitalisations than children with neither condition, and this effect persisted after taking into account birth year and preterm birth status. While overall hospitalisation rates differ by age, the rate of hospitalisation appears to decline with increasing age, particularly for the groups with a known biomedical cause and with severe ID of unknown cause, in contrast to the general population where the rate appears more constant. We also showed that the independent effect of preterm birth on hospitalisation rates after 1 year of age is both an increase in frequency and earlier median age at hospitalisation, and this is consistent across all case groups.
Recent studies in the USA17 and Denmark22 have investigated the hospitalisation burden for children with autism, and found that individuals with autism had significantly higher lengths of stay and that costs were considerably more than for those without autism. The Danish study also found that children with ASD had an increased rate of contact with the hospital, regardless of the cause of their hospital admission. However, unlike our study, neither of these two studies were able to compare the hospitalisation patterns of children with ASD with those of children with ID. What we found was that the highest rate of hospitalisations occurred among the severe ID group, followed by those with a known biomedical cause for their ID, mild/moderate ID, ASD with ID, ASD without ID and then the comparison population. Thus, the rate of hospitalisation was positively correlated with the severity of ID. Our study was able to demonstrate the gradient of risk between the groups over the study period using the same data methods, whole-of-population sampling and controlling for interaction variables. We have also shown an increasing discrepancy in hospitalisations over time between those with severe ID and no ASD and those with a biomedical cause, particularly at younger ages. One possible explanation is that, in more recent years, the aetiology of severe ID is being increasingly identified through new genetic techniques,25 26 and thus there has been a diagnostic transfer from those with severe ID of unknown cause to those with a biomedical cause over time. Another explanation may be the improved survival in those with severe ID of unknown cause which is associated with ongoing high medical needs.
Various comorbid health conditions affect children with ID, including epilepsy, skin conditions, sensory loss, fractures and psychiatric disorders.2–4 However, apart from individual conditions such as Down syndrome,6 Rett syndrome,27 Tuberous Sclerosis28 and Angelman syndrome29 and a recent paper describing the increase over time in resource utilisation by children with neurological impairment,30 there is a dearth of population-based data on medical comorbidities associated with ID. Our results would suggest that, although ID may be under-recognised and under-researched in comparison to autism, its associated health burden should not be underestimated. In Canada, it has been shown that, for ambulatory care sensitive conditions, people with an ID are hospitalised at six times the rate of those without an ID, with the rate ratio peaking at double this for those aged 30–39 years.31 This may reflect a lack of appropriate and accessible primary care for people with ID, particularly for those transitioning from the paediatric healthcare system. The highest hospitalisation rate ratio was for epilepsy, known to be a common comorbid condition for people with ID,3 and a condition with a high level of impact on the life of the individual and their family.32
As with ID, various comorbid health conditions including gastrointestinal disorders, sleep disturbances, sensory impairments, diabetes mellitus type 1 and epilepsy also occur in ASD.9 16 33 34 Although the hospitalisation burden for children with ASD17 35 has previously been reported to be high, we have clearly shown in our present study that for children with ID the burden is considerably greater. Behaviour and communication difficulties are reported to be significant barriers to utilising hospital care for children with developmental disabilities19 36 and may increase the overall burden and amount of family support necessary.37 38 Such challenging behaviours are known to be more frequent in children with ASD than in those with ID and thus may increase the difficulty in their accessing hospital care. Comorbid psychopathology is particularly common in children with ASD,39 and the specific types of behaviours associated with ASD may discourage hospitalisation and also shorten the length of stay.40 More detailed research on the patterns of primary and secondary healthcare use for people with an ID and/or ASD, according to health burden and sociodemographic status, is needed to unpack the associations between these disorders and increased hospitalisation. Owing to the increased risk of hospitalisations of children with ID and ASD, resource planners need to prioritise specialised care allocation for these populations. Lack of medical and nursing staff trained in developmental disability management skills may, in the past, have led to negative experiences, inadequate care and compounded problems.41 42 Qualitative information from parents would help to understand these relationships and identify specific factors that may affect or modify the risk of hospitalisation in these children. Such research would be important in helping to inform resource planning. We concur with the plea made by Berry et al30 to ensure that the current healthcare system is adequately educated and equipped to care for this growing proportion of vulnerable children within the hospital population.
The strength of our study has been the ability to use population-based data on children's hospitalisations and link these to sources of disability diagnosis (ie, presence of ID or ASD). By including birth year in the model, we were able to account for these changes over time and show how the patterns of risk of hospitalisation changed for individuals in the different categories. We also accounted for possible confounding by preterm birth status, known to be associated with increased childhood hospitalizations,43 and demonstrated the consistent effect of preterm birth on hospitalisation rates across all case groups and the rest of the population. Weaknesses in our study included exclusion from our modelling of hospitalisation history in the first year of life, which may indeed change later risk of hospitalisation. This was lack of access to birth date data (for privacy reasons) and to a change in coding practices in the WA healthcare system that occurred in the mid-1990s.
Children with developmental disabilities have an increased risk of hospitalisation, the extent of which varies according to the type of disability and level of intellectual functioning. Future research should investigate how hospitalisations relate to underlying morbidities, common in ID and less so in autism, and also consider the role of access to primary care in preventing unnecessary hospitalisation. A better understanding of the patterns of hospitalisation for these children will help establish resource planning opportunities for the specific services required to meet their increasing needs.44
Supplementary Material
Acknowledgments
The authors are grateful to the Disability Services Commission, the Telethon Institute for Child Health Research, the Western Australia Department of Education, the Catholic Education Office and the Association of Independent Schools of Western Australia for assistance with data collection for the IDEA database. We thank Dr Geoff Hammond for his statistical advice on aspects of the paper.
Footnotes
Contributors: AB has contributed to the paper through involvement in study design, statistical analysis and interpretation and manuscript preparation. EG, JB and HL have contributed to the paper through involvement in study design, manuscript editing and revision and final review.
NdK has contributed to the paper through statistical design and interpretation and final manuscript review.
Funding: This study was supported by Program Grant #572742 from the Australian National Health and Medical Research Council (NHMRC) and NHMRC Senior Research Fellowship #572742 (Dr Helen Leonard).
Competing interests: None.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: There are no additional data available.
References
- 1.Leonard H, Petterson B, Bower C, et al. Prevalence of intellectual disability in Western Australia. Paediatr Perinat Epidemiol 2003;17:58–67 [DOI] [PubMed] [Google Scholar]
- 2.Jansen DE, Krol B, Groothoff JW, et al. People with intellectual disability and their health problems: a review of comparative studies. J Intell Disabil Res 2004;48(Pt 2):93–102 [DOI] [PubMed] [Google Scholar]
- 3.Oeseburg B, Dijkstra GJ, Groothoff JW, et al. Prevalence of chronic health conditions in children with intellectual disability: a systematic literature review. Intellect Dev Disabil 2011;49:59–85 [DOI] [PubMed] [Google Scholar]
- 4.van Schrojenstein Lantman-de Valk HMJ. Health in people with intellectual disabilities: current knowledge and gaps in knowledge. J Appl Res Intellect Disabil 2005;18:325–33 [Google Scholar]
- 5.Leonard H, Wen X. The epidemiology of mental retardation: challenges and opportunities in the new millennium. Ment Retard Dev D R 2002;8:117–34 [DOI] [PubMed] [Google Scholar]
- 6.Thomas K, Girdler S, Bourke J, et al. Overview of health issues in school-aged children with down syndrome. In: Urbano RC, ed. Int Rev Res Ment Ret. Academic Press, 2010:67–106 [Google Scholar]
- 7.Cassidy SB, Schwartz S, Miller JL, et al. Prader-Willi Syndrome. Genet Med 2012;14:10–26 Epub 2012/01/13 [DOI] [PubMed] [Google Scholar]
- 8.Williams K, Leonard H, Tursan d'Espaignet E, et al. Hospitalisations from birth to 5 years in a population cohort of Western Australian children with intellectual disability. Arch Dis Child 2005;90:1243–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schieve LA, Gonzalez V, Boulet SL, et al. Concurrent medical conditions and health care use and needs among children with learning and behavioral developmental disabilities, National Health Interview Survey, 2006–2010. Res Dev Disabil 2012;33:467–76 [DOI] [PubMed] [Google Scholar]
- 10.Einfeld SL, Tonge BJ. Population prevalence of psychopathology in children and adolescents with intellectual disability: II. Epidemiological findings. J Intell Disabil Res 1996;40(Pt 2):99–109 [DOI] [PubMed] [Google Scholar]
- 11.Balogh RS, Hunter D, Ouellette-Kuntz H. Hospital utilization among persons with an intellectual disability, Ontario, Canada, 1995–2001. J Appl Res Intellect Disabil 2005;18:181–90 [Google Scholar]
- 12.American Psychiatric Association Diagnostic and statistical manual of mental health disorders 4th edition. Washington, DC: American Psychiatric Association, 1994 [Google Scholar]
- 13.Leonard H, Dixon G, Whitehouse A, et al. Unpacking the complex nature of the autism epidemic. Res Autism Spect Dis 2010;4:548–54 [Google Scholar]
- 14.Baio J. Prevalence of autism spectrum disorders-Autism and Developmental Disabilities Monitoring Network, 14 Sites, United States 2008. MMWR CDC Surveill Summ 2012;61:1–19 [PubMed] [Google Scholar]
- 15.Leonard H, Glasson E, Nassar N, et al. Autism and intellectual disability are differentially related to sociodemographic background at birth. PLoS ONE 2011;6:e17875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kohane IS, McMurry A, Weber G, et al. The co-morbidity burden of children and young adults with autism spectrum disorders. PLoS ONE 2012;7:e33224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lokhandwala T, Khanna R, West-Strum D. Hospitalization burden among individuals with autism. J Autism Dev Disord 2012;42:95–104 [DOI] [PubMed] [Google Scholar]
- 18.Thomas K, Bourke J, Girdler S, et al. Variation over time in medical conditions and health service utilization of children with Down syndrome. J Pediatr 2011;158:194–200 e1 [DOI] [PubMed] [Google Scholar]
- 19.Lunsky Y, Lin E, Balogh R, et al. Are adults with developmental disabilities more likely to visit EDs? Am J Emerg Med 2011;29:463–5 [DOI] [PubMed] [Google Scholar]
- 20.Lunsky Y, Balogh R, Khodaverdian A, et al. A comparison of medical and psychobehavioral emergency department visits made by adults with intellectual disabilities. Emerg Med Int 2012;2012:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Petterson B, Leonard H, Bourke J, et al. IDEA (Intellectual Disability Exploring Answers): a population-based database for intellectual disability in Western Australia. Ann Hum Biol 2005;32:237–43 [DOI] [PubMed] [Google Scholar]
- 22.Leonard H, Petterson B, De Klerk N, et al. Association of sociodemographic characteristics of children with intellectual disability in Western Australia. Soc Sci Med 2005;60:1499–513 [DOI] [PubMed] [Google Scholar]
- 23.Yeargin-Allsopp M, Murphy CC, Cordero JF, et al. Reported biomedical causes and associated medical conditions for mental retardation among 10-year-old children, metropolitan Atlanta, 1985 to 1987. Dev Med Child Neurol 1997;39:142–9 [DOI] [PubMed] [Google Scholar]
- 24.Holman CD, Bass AJ, Rosman DL, et al. A decade of data linkage in Western Australia: strategic design, applications and benefits of the WA data linkage system. Aust Health Rev 2008;32:766–77 [DOI] [PubMed] [Google Scholar]
- 25.Jaillard S, Drunat SV, Bendavid C, et al. Identification of gene copy number variations in patients with mental retardation using array-CGH: novel syndromes in a large French series. Eur J Med Genet 2010;53:66–75 [DOI] [PubMed] [Google Scholar]
- 26.Koolen DA, Pfundt R, de Leeuw N, et al. Genomic microarrays in mental retardation: a practical workflow for diagnostic applications. Hum Mutat 2009;30:283–92 [DOI] [PubMed] [Google Scholar]
- 27.Young D, Bebbington A, de Klerk N, et al. The relationship between MECP2 mutation type and health status and service use trajectories over time in a Rett syndrome population. Res Autism Spect Dis 2011;5:442–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Osborne JP, Merrifield J, O'Callaghan FJ. Tuberous sclerosis—what's new? Arch Dis Child 2008;93:728–31 [DOI] [PubMed] [Google Scholar]
- 29.Williams CA, Driscoll DJ, Dagli AI. Clinical and genetic aspects of Angelman syndrome. Genet Med 2010;12:385–95 [DOI] [PubMed] [Google Scholar]
- 30.Berry JG, Poduri A, Bonkowsky JL, et al. Trends in resource utilization by children with neurological impairment in the United States inpatient health care system: a repeat cross-sectional study. PLoS Med 2012;9:e1001158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Balogh R, Brownell M, Ouellette-Kuntz H, et al. Hospitalisation rates for ambulatory care sensitive conditions for persons with and without an intellectual disability—a population perspective. J Intell Disabil Res 2010;54:820–32 [DOI] [PubMed] [Google Scholar]
- 32.Bowley C, Kerr M. Epilepsy and intellectual disability. J Intell Disabil Res 2000;44(Pt 5):529–43 [DOI] [PubMed] [Google Scholar]
- 33.Lauritsen MB, Mors O, Mortensen PB, et al. Medical disorders among inpatients with autism in Denmark according to ICD-8: a nationwide register-based study. J Autism Dev Disord 2002;32:115–19 [DOI] [PubMed] [Google Scholar]
- 34.Bolton PF. Medical conditions in autism spectrum disorders. J Neurodev Disord 2009;1:102–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Atladottir HO, Schendel D, Lauritsen M, et al. Patterns of contact with hospital for children with an autism spectrum disorder: a Danish Register-Based Study. J Autism Dev Disord 2012;42:1717–28 [DOI] [PubMed] [Google Scholar]
- 36.van Schrojenstein Lantman-de Valk, van den Akker HMJ, Maaskant MMA, et al. Prevalence and incidence of health problems in people with intellectual disability. J Intell Disabil Res 1997;41:42–51 [DOI] [PubMed] [Google Scholar]
- 37.Hemsley B, Balandin S, Togher L. Narrative analysis of the hospital experience for older parents of people who cannot speak. J Aging Stud 2007;21:239–54 [Google Scholar]
- 38.Iacono T, Davis R. The experiences of people with developmental disability in emergency departments and hospital wards. Res Dev Disabil 2003;24:247–64 [DOI] [PubMed] [Google Scholar]
- 39.Brereton AV, Tonge BJ, Einfeld SL. Psychopathology in children and adolescents with autism compared to young people with intellectual disability. J Autism Dev Disord 2006;36:863–70 [DOI] [PubMed] [Google Scholar]
- 40.Scarpinato N, Bradley J, Kurbjun K, et al. Caring for the child with an autism spectrum disorder in the acute care setting. J Spec Pediatr Nurs 2010;15:244–54 [DOI] [PubMed] [Google Scholar]
- 41.Lennox N, Diggens J. Knowledge, skills and attitudes: Medical schools’ coverage of an ideal curriculum on intellectual disability. J Intellect Dev Dis 1999;24:341 [Google Scholar]
- 42.Ouellette-Kuntz H. Understanding health disparities and inequities faced by individuals with intellectual disabilities. J Appl Res Intellect Disabil 2005;18:113–21 [Google Scholar]
- 43.Petrou S, Mehta Z, Hockley C, et al. The impact of preterm birth on hospital inpatient admissions and costs during the first 5 years of life. Pediatrics 2003;112(6 Pt 1):1290–7 [DOI] [PubMed] [Google Scholar]
- 44.Brownell MD, Derksen SA, Jutte DP, et al. Socio-economic inequities in children's injury rates: has the gradient changed over time? Can J Public Health 2010;101(Suppl 3):S28–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.