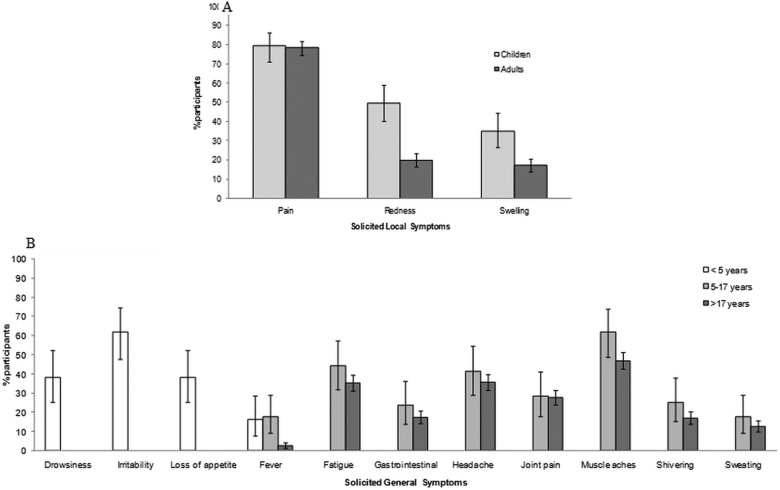
Figure 2.
Solicited local (A) and general (B) adverse events reported during a 7-day follow-up period after any dose (Reactogenicity cohort N=682). The general symptoms of drowsiness, irritability and loss of appetite were only assessed in children <5 years, whereas fatigue, gastrointestinal, headache, joint pain, muscle aches, shivering and sweating were assessed in children aged 5–17 years and in adults. Fever was defined as an oral or axillary temperature of ≥37.5°C (99.5°F) or a rectal temperature of ≥38.0°C (100.4°F). Data are shown as the percentage of participants reporting the symptom with 95% CI.