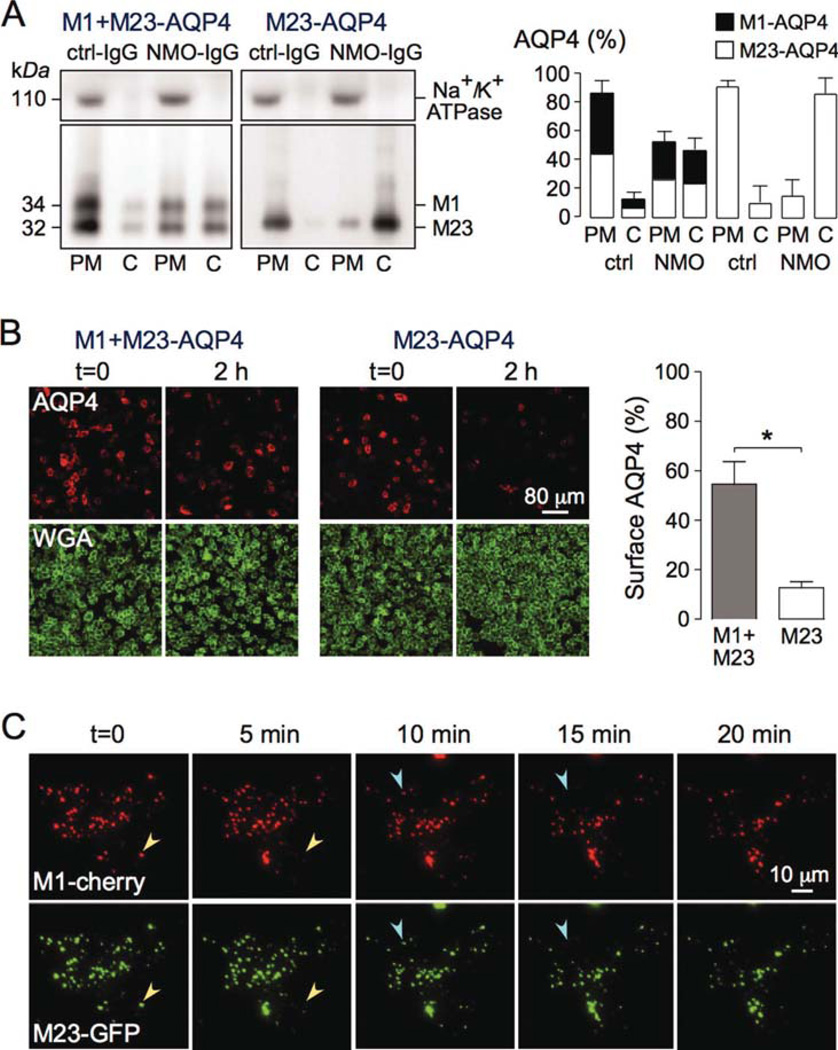
Fig. 5.
NMO-IgG binding causes simultaneous cointernalization of M1- and M23-AQP4 when coexpressed in transfected cells. (A) CHO cells were transiently transfected with M23-AQP4 only or together with an equal amount of M1-AQP4, and incubated with 50 µg/mL NMO-IgG (rAb-58) or control-IgG (ctrl-IgG) for 2 h at 37°C. (Left) Immunoblot of AQP4 and Na+/K+ ATPase following SDS/PAGE of plasma membrane (PM) and intracellular vesicles (cytoplasmic, C) fractions. (Right) M1- and M23-AQP4 amounts from blots as in A (SE, n = 3). (B) (Left) Cells treated as in A were stained for surface AQP4 (red) and plasma membrane (WGA, green). (Right) Percentage of remaining AQP4 at the cell surface after 2 h incubation with NMO-IgG (SE, n = 10, *P < 0.01). Representative of two sets of experiments. (C) TIRFM of U87MG cells transfected with M1-mCherry (red) and M23-GFP (green), incubated with 50 µg/mL rAb58 for 1 h at 4°C, and then chased at 37°C for indicated times (full time course in Supp. Info. Movie 2). Arrowheads of the same color in consecutive micrographs show internalized OAPs containing both isoforms. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]