Abstract
Successful cryopreservation of functional engineered tissues (ETs) is significant to tissue engineering and regenerative medicine, but it is extremely challenging to develop a successful protocol because the effects of cryopreservation parameters on the post-thaw functionality of ETs are not well understood. Particularly, the effects on the microstructure of their extracellular matrix (ECM) have not been well studied, which determines many functional properties of the ETs. In this study, we investigated the effects of two key cryopreservation parameters – i) freezing temperature and corresponding cooling rate; and ii) the concentration of cryoprotective agent (CPA) on the ECM microstructure as well as the cellular viability. Using dermal equivalent as a model ET and DMSO as a model CPA, freezing-induced spatiotemporal deformation and post-thaw ECM microstructure of ETs was characterized while varying the freezing temperature and DMSO concentrations. The spatial distribution of cellular viability and the cellular actin cytoskeleton was also examined. The results showed that the tissue dilatation increased significantly with reduced freezing temperature (i.e., rapid freezing). A maximum limit of tissue deformation was observed for preservation of ECM microstructure, cell viability and cell-matrix adhesion. The dilatation decreased with the use of DMSO, and a freezing temperature dependent threshold concentration of DMSO was observed. The threshold DMSO concentration increased with lowering freezing temperature. In addition, an analysis was performed to delineate thermodynamic and mechanical components of freezing-induced tissue deformation. The results are discussed to establish a mechanistic understanding of freezing-induced cell-fluid-matrix interaction and phase change behavior within ETs in order to improve cryopreservation of ETs.
Keywords: Cryopreservation, Tissue microstructure, Extracellular matrix, Cell image deformetry, Differential scanning calorimetry, Cryoprotective agents
1. Introduction
As tissue engineering and regenerative medicine progress, a wide variety of engineered tissue products have been developed and need to be preserved in order to maintain operational stock for distribution and use (Mansbridge 2006; Nerem 2006; Nerem 2010). Although several methods have been proposed, cryopreservation, which preserves tissues in the frozen state with cryoprotective agents, remains as the primary candidate for long-term storage of tissues (Karlsson and Toner 1996; Pegg 2002). Successful cryopreservation of a few types of native and engineered tissues has been reported (Aggarwal et al. 1985; Ishine et al. 2000; Elder et al. 2005), but cryopreservation is still restricted in yielding consistent and reliable preservation outcomes, especially in preserving tissue functionalities. Maintenance of post-thaw tissue functionalities is critical for successful cryopreservation of engineered tissues. Since many tissue functionalities are associated with the microstructure of the extracellular matrix (ECM), both the ECM microstructure as well as cellular viability should be maintained during cryopreservation procedures. In spite of their significance, preserving both ECM microstructure and cellular viability have not been properly considered during the development of cryopreservation protocols.
Freezing-induced cellular injuries have been explained by two-factor hypotheses, which include extracellular and intracellular ice formation (IIF) and solution effects (Mazur 1984; Karlsson et al. 1993; Muldrew et al. 2000; Han and Bischof 2004). Compared to cellular-level cryoinjury, the effects of freezing on the ECM microstructure have not been well understood, though tissue level injuries are equally important to investigate. Recent studies (Han et al. 2009; Teo et al. 2010; Teo et al. 2011) have shown that spatiotemporal tissue deformation occurs during freezing and that it determines the post-thaw ECM microstructure. These studies have also demonstrated that freezing induces complex interactions among cells, interstitial fluid and the ECM. These interactions include volumetric expansion of the ECM due to water/ice phase change, compression of the adjacent unfrozen ECM, interstitial fluid transport from the freezing interface into the unfrozen ECM, and mechanical stress/strain interaction between cells and the ECM. Although these cell-fluid-matrix interactions are thought to affect the post-thaw ECM microstructure and cellular viability, the effects of cryopreservation protocol parameters on these interactions are not well characterized.
Several parameters of cryopreservation protocols have been shown to affect cell survival as well as tissue functional properties (Song et al. 1994; David et al. 2001; Pegg 2002; Devireddy et al. 2003). These parameters include freezing temperature, cooling and warming rates, and type and concentration of the cryoprotective agent (CPA). Typically, these parameters have been investigated and empirically optimized for specific cell and tissue types to achieve improved cellular survival. CPAs such as dimethyl sulfoxide (DMSO) are frequently used in cryopreservation procedures to improve the preservation outcomes. CPAs have been shown to improve the outcome of cryopreservation by maintaining tissue structural integrity and cellular viability (Neidert et al. 2004; Kubo and Kuroyanagi 2005; Schenke-Layland et al. 2007; Wang et al. 2007). However, mechanistic understanding of the effects of cryopreservation parameters on post-thaw ECM microstructure is still lacking. This significantly hinders the development of preservation protocols for new types of engineered tissues.
The objective of the current study is to test a hypothesis that the freezing-induced tissue deformation should be limited below a tissue-specific maximum in order to preserve the ECM microstructure and cell viability. It is also hypothesized that to achieve this acceptable ECM microstructure and cell viability, a freezing temperature-dependent threshold CPA concentration is required, which keeps the tissue deformation below that maximum. This tissue-specific mechanical behavior of maximum deformation is thought to be caused by complex fluid-matrix interactions during freezing. To measure the freezing-induced spatiotemporal deformation of the ET, an experimental technique called quantum dot-mediated Cell Image Deformetry (CID) (Teo et al. 2010) is used. The post-thaw ECM microstructure was characterized using scanning electron microscopy (SEM). After freeze/thaw (F/T), the spatial distribution of cellular viability was determined, and the cellular actin cytoskeleton structure was examined using an immunofluorescence technique. The freezing-induced tissue deformation is thought to be contributed by two factors, namely, phase change-mediated thermodynamic deformation and fluid-matrix interaction-instigated mechanical deformation. To delineate these two factors and to explain the existence of the temperature-dependent threshold CPA concentration, differential scanning calorimetry (DSC) was used to investigate the thermodynamic freezing response of the ETs by varying temperature and CPA concentration. The results are discussed from the perspective of how each component in a tissue – cells, extracellular matrix, and interstitial fluid – interacts with the others during freezing. These results help to establish understanding of biophysical phenomena during tissue cryopreservation, and lay the groundwork to link cryopreservation parameters to post-thaw tissue functionalities.
2. Materials and methods
2.1 Cell culture and engineered tissues
Human dermal fibroblasts were cultured in Dulbecco’s modified Eagle medium (DMEM/F12, Invitrogen, Grand Island, NY) supplemented with 10% fetal bovine serum, 2 mM L-glutamine, and 100 μg/mL penicillin/streptomycin. The fibroblasts were maintained in 75 cm2 T-flasks at 37 °C and 5% CO2. By using 0.05% trypsin with 0.53 mM EDTA, the cells were consistently harvested at 80–90% confluency between the sixth and fifteenth passages. The preparation of an engineered tissue (ET) using type I collagen (BD Biosciences, Bedford, MA) has been described previously (Teo et al. 2010). Briefly, fibroblasts were suspended in a collagen solution (3.0 mg-collagen/mL) at a concentration of 2 × 105 cells/mL. The mixture of cells and collagen solution was then polymerized in a chamber slide (Thermo Scientific Nunc, Naperville, IL) for 60 minutes at 37 °C. After polymerization, 2 mL of culture medium was added. The ETs were then incubated for 24 hours prior to the experiments.
2.2 CPA treatment of engineered tissues
Before the freezing experiments, CPA-treated ET groups were prepared by loading 1, 2, and 3 M DMSO (Sigma Aldrich, St. Louis, MO), respectively. The range of DMSO concentrations was determined relevant to typical cryopreservation medium (10% v/v DMSO) and also relevant to recent interest in using high DMSO concentration to cryopreserve tissues (Neidert et al. 2004; Kubo and Kuroyanagi 2005; Schenke-Layland et al. 2007; Wang et al. 2007). In order to minimize osmotic shock, the ETs were suspended in gradually increasing concentrations of DMSO (i.e., 0.25, 0.5, 0.75, 1, 1.5, 2.0, 2.5, and 3.0 M), each of which lasted for 15 minutes to achieve complete permeation of DMSO throughout the ETs. The cytotoxic effect of DMSO and the osmotic stresses induced by loading and unloading of the DMSO are found to be minimal, and the ETs retained their cell viability at approximately 90% after the DMSO treatment.
2.3 Cell image deformetry using engineered tissues with quantum dot-labeled fibroblasts
The spatiotemporal deformation of ETs during freezing was measured using Cell Image Deformetry (CID). Measuring tissue deformation during freezing by using CID has been described elsewhere (Teo et al. 2010). For this experiment, ETs were prepared with fibroblasts labeled with cell-targeted quantum dots (Qtracker 655 Cell-Labeling Kit, Invitrogen, Eugene, OR). After 24-hour incubation, the ETs were frozen on a directional solidification stage while being imaged using a CCD camera (Retiga-2000R, Q-Imaging, Surrey, BC) coupled to a fluorescence microscope (MVX10, Olympus, Melville, NY). A temperature gradient ranging from TH = 4 °C to a sub-zero temperature, TL (i.e., −20, −40, or −60 °C), over a distance of 6 mm was imposed on the ETs, causing the ETs to freeze uni-directionally. This directional freezing was designed to mimic the freezing process experienced by tissues in a controlled-rate freezer, where the samples are exposed to a freezing temperature in a chamber. The temperature of this chamber is programmed to be lowered at desired cooling rates. Even though the chamber temperature decreases temporally, the freezing in the sample begins from the outer boundary of the sample and propagates into the interior. In order to mimic this clinical directional freezing process under a microscope, directional freezing was imposed with a predetermined freezing temperature as illustrated in Fig. 1. As a result of this directional freezing, the cooling rate changes spatiotemporally as illustrated in Fig. S1 and table ST2. From the table it can be noted that the spatiotemporal cooling rate is also varying, and its range is approximately within 0.69 °C/ min to 42.5 °C/ min. Therefore, the freezing temperature was varied to create different spatiotemporal cooling rates. Thus, cooling rate and freezing temperature are dependent quantities. But it should be noted that for a given freezing temperature, the cooling rate at different locations also varies as evident from the table. To avoid reporting all the cooling rates for each individual case, the ‘freezing temperature’ is the single invariant parameter reported here.
Fig. 1.
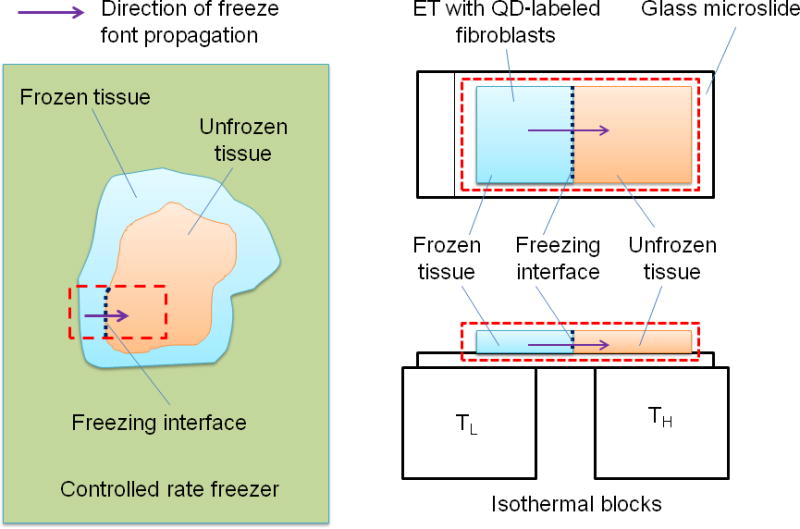
Schematic of the freezing procedure: The left side shows a typical procedure of tissue cryopreservation. The native/ engineered tissues are frozen inside a controlled-rate freezer. The freeze front propagates from the outside boundary to the interior of the tissue as shown by the bold arrow. This procedure is mimicked using a directional solidification stage. The tissue is placed on a glass microslide which rests on the cold and hot isothermal posts. Because of the temperature gradient created, the freeze front propagates along the direction shown by the bold arrow.
Images acquired during freezing were cross-correlated to estimate local deformation rates in the x and y directions, u and v. Area dilatation was then computed as follow:
| (1) |
In order to characterize the freezing conditions, the location of the freezing interface, X(t), is monitored and curve-fitted using the analytical Neumann solution for phase change as below:
| (2) |
where λ is a parameter characterizing the given freezing conditions (i.e., freezing interface velocity), and αs is the thermal diffusivity of ice (1 × 106 μm2/s).
2.4 Scanning electron microscopy
The post-thaw ECM microstructure was visualized using scanning electron microscopy (SEM) as previously described in (Teo et al. 2011). After F/T, circular sections, 3 mm in diameter, were punched out from the frozen/thawed and unfrozen regions of the ETs. The tissue sections were fixed with 1% tannic acid for 1 minute, followed by staining with 2% uranyl acetate for 20 minutes. The tissue sections were then placed into wet-SEM sample holders (QX-302, Quantomix, Hartfield, PA) with the addition of 10 mL buffering solution (QX-302 imaging buffer, Quantomix). The samples were imaged in the hydrated state using a scanning electron microscope (Quanta 3D FEG DualBeam, FEI, Hillsboro, OR).
2.5 Post-thaw cell viability
Cell viability was evaluated 3 hours after F/T and unloading of the CPA by using a membrane integrity assay. Cells in the ETs were stained with Hoechst 33342 (Sigma Aldrich, St. Louis, MO) and propidium iodide (Sigma Aldrich, St. Louis, MO) for 30 minutes at 37 °C. All cells and necrotic (non-viable) cells were counted under a fluorescence microscope, and cell viability was evaluated with respect to the control (i.e., unfrozen ET that was not treated with DMSO) as given in the following equation (Han et al. 2005):
| (3) |
2.6 Immunofluorescence microscopy
Post-thaw tissue samples were fixed with 3% paraformaldehyde (Ted Pella, Redding, CA), followed by a 30-minute treatment with 2% BSA (Fraction V, Equitech-Bio, Kerrville, TX) and 1% glycine (Sigma Aldrich, St. Louis, MO) to block non-specific staining. The samples were then permeabilized with 0.5% Triton X-100 (Sigma Aldrich, St. Louis, MO) for 15 minutes. For actin staining, the samples were incubated with Alexa-Fluor 488 conjugated phalloidin (1:150 dilution in 1% BSA/DPBS) at 37 °C for 30 minutes. The samples were mounted on glass microslides with Fluoromount-G (Southern Biotech, Birmingham, AL). Confocal images of the samples were captured using an OptiGrid structured-illumination microscope system (Qioptiq, Fairport, NY).
2.7 Thermodynamic analysis of freezing-induced expansion
In addition to the CID experiments, in order to characterize the effects of freezing temperature and DMSO concentration on the freezing-induced volumetric expansion associated with water/ice phase change, the latent heat release patterns of the ETs were characterized using a differential scanning calorimeter (DSC Q200, TA Instruments, New Castle, DE). Approximately 4–6 mg of each sample were placed in an aluminum pan that was hermetically sealed with an aluminum lid. The sample was pre-nucleated by supercooling and then reheated to 0.2 °C below its ice melting temperature. The sample was held at this temperature for two minutes so that only a small amount of ice was present as nuclei in the sample. After the pre-nucleation, the sample was cooled to −50 °C at 1 °C/min, during which exothermic heat flow was measured. The latent heat release pattern with respect to temperature, L(T), was then obtained by integrating the exothermic heat flow thermogram of each sample. Since the latent heat is only associated with the water/ice phase change, the ratio of L(T) to the total latent heat release, Ltotal, is proportional to the frozen fraction of the sample at a given temperature T. The corresponding volumetric expansion with respect to initial volume, V(T), was thermodynamically estimated as below considering the porosity of the ETs, φ, and the density of water, ρl, and ice, ρs.
| (4) |
Then, the volumetric expansion rate was computed as follows:
| (5) |
The first term on the right hand side of equation (2) was determined from equation (1), and dT/dt was determined from the local temperature history measured. The dV/dt term is called the thermodynamic temporal expansion rate, in contrast to the dilatation, e, which was measured using CID and is a measure of the spatiotemporal deformation of the ETs during freezing.
2.8 Statistical analysis
Each experimental group was repeated at least three times (n ≥ 3). All numerical results are presented as mean ± standard error of the mean. Statistical analysis was performed using one-way ANOVA with a P-value less than 0.05 considered statistically significant.
3. Results
3.1 Effects of freezing temperature on tissue deformation
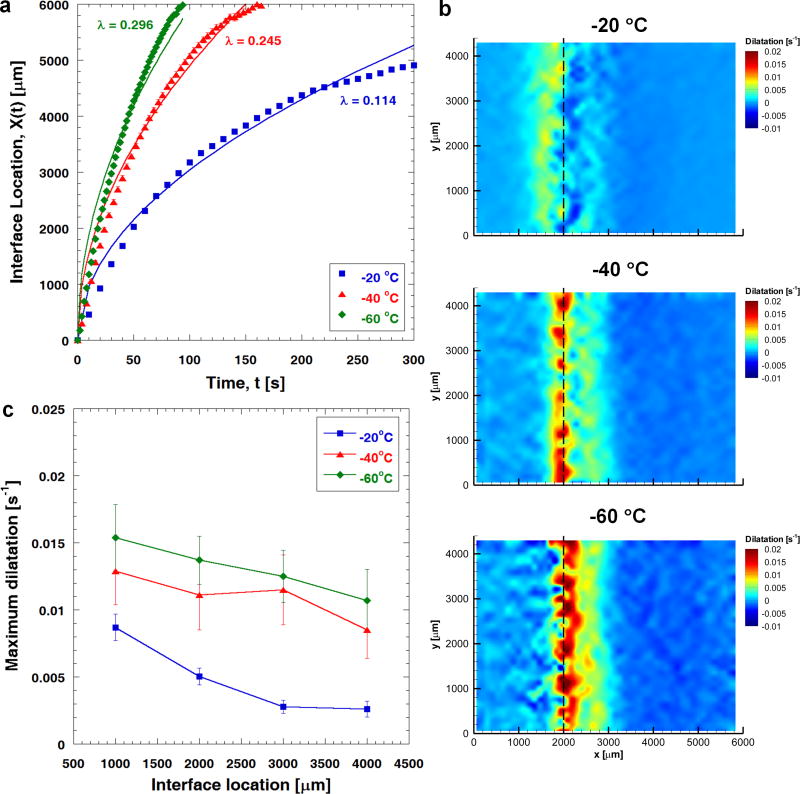
Fig. 2a summarizes the effects of freezing temperature on the phase change interface propagation during freezing. The phase change interface location, X(t), is plotted against time, t, and is curve-fitted using , in which the parameter λ represents the freezing conditions experienced by the ETs. As shown in Fig. 2a, a lower freezing temperature causes the freezing interface to propagate further into the unfrozen region, and leads to faster freezing interface propagation as indicated by a higher value of λ. When TL = −20 °C, the terminal freezing interface location is at about 5000 μm, and the value of λ is approximated to be 0.114 (R2 = 0.982). As TL decreases, the terminal freezing interface location changes to 6000 μm, and λ increases to 0.245 (TL = −40 °C; R2 = 0.966) and 0.296 (TL = −60 °C; R2 = 0.947), respectively.
Fig. 2.
Effects of freezing temperature on freezing-induced tissue deformation (n=3). (a) Phase change interface location during freezing and its curve fit using . Lowering freezing temperature leads to faster freezing interface propagation, resulting in a higher value of λ. The terminal interface location is at approximately 5000 μm when TL = −20 °C, and 6000 μm when TL = −40 and −60 °C. (b) Local dilatations experienced by engineered tissues when X(t) = 2000 μm for freezing temperatures of −20, −40 and −60 °C, respectively. Local dilatation increases in magnitude with lowering freezing temperature. The location of the maximum dilatation shifts closer to the freezing interface into the unfrozen region as the freezing temperature decreases. (c) The magnitude of the maximum dilatation for each freezing temperature. Note that the freezing interface location, X(t), is proportional to .
The effects of freezing temperature on freezing-induced tissue deformation are shown in Fig. 2b and 2c. The local dilatations experienced by the ETs when the freezing interface is located at 2000 μm are shown in Fig. 2b (n = 3) for the various tissue freezing temperatures. The tissue deformation pattern changes significantly, and the magnitude of the dilatation increases with lowering freezing temperature. When X(t) ≈ 2000 μm, as TL decreases from −20 °C to −60 °C, the maximum local dilatation measured in the ETs increases by 170% from 0.0050 ± 0.0006 s−1 to 0.0137 ± 0.0017 s−1. In addition, the location of the maximum deformation changes with freezing temperature. When TL = −20 °C, the maximum deformation occurs at the freezing interface. This results in a local expansion just after the phase change interface, along with a local compression in the unfrozen region adjacent to the interface. However, as the freezing temperature decreases, the location of the maximum deformation shifts further into the unfrozen region adjacent to the freezing interface. This leads to the local expansion occurring right at the freezing interface.
3.2 Effects of freezing temperature on ECM microstructure, cell viability and cytoskeletal structure
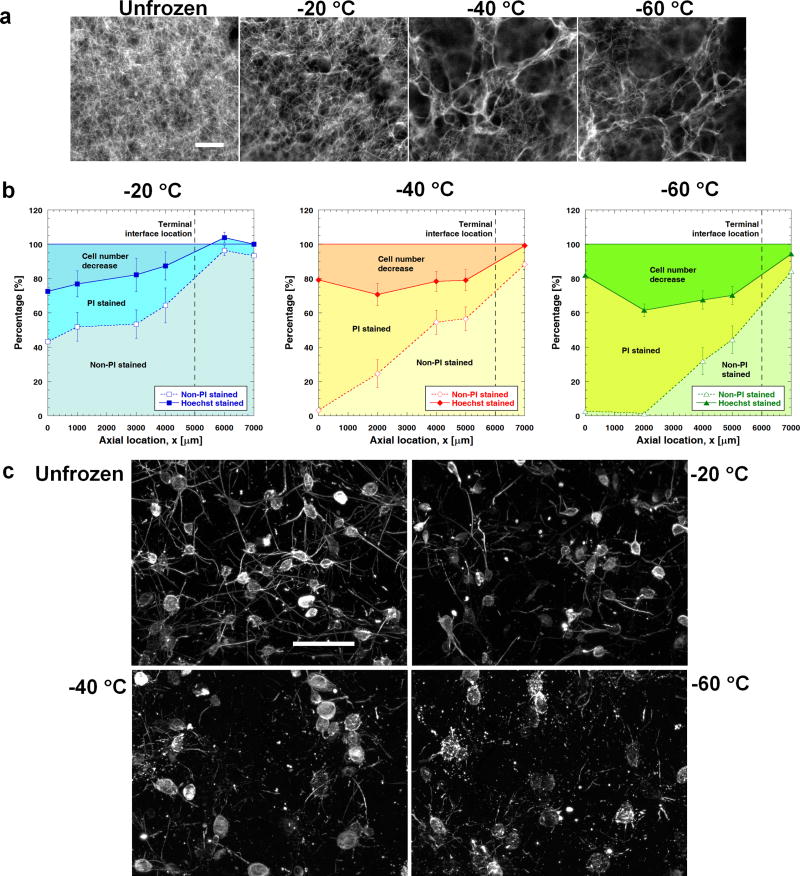
The effects of freezing temperature on post-thaw ECM microstructure are shown in Fig. 3a. The void space (i.e., the size of the pores) increases noticeably as the freezing temperature decreases. These results are consistent with the tissue deformation measurements. The dilatation experienced by the ETs becomes larger when frozen to a lower temperature (see Fig. 2b). Fig. 3b summarizes the effects of freezing temperature on post-thaw cell viability at different axial locations in the ETs. It is assumed that the initial cell distribution is uniform and similar to the control (i.e., unfrozen ET that is not treated with DMSO). In the experiment, all adherent cells are indicated by Hoechst staining, live cells (i.e., with intact membrane) by non-PI staining, cells with membrane damage by PI staining, and cell detachment is reflected by the decrease in cell number. As shown in Fig. 3b, the number of cells experiencing membrane damage increases as a result of lowering freezing temperature. At the same time, the number of detached cells also increases. These findings are in accordance with the tissue deformation results, in which the dilatation measured in the ETs during freezing increases with lowering freezing temperature. Fig. 3c shows the effects of different freezing temperatures on the cell-matrix adhesion. In the unfrozen region, long and thin cellular extensions are clearly observed. After F/T, these cellular extensions are noticeably broken, and they are fragmented into specks when the freezing temperature decreases to −60 °C. Apparently, more cell-matrix bindings are damaged as a result of lowering freezing temperature. This explains the increased number of detached cells shown in Fig. 3b when the freezing temperature decreases.
Fig. 3.
Effects of freezing temperature on ECM structure, cell viability and actin cytoskeletal structure. (a) Scanning electron micrographs of unfrozen and frozen/thawed ECM microstructures for different freezing temperatures (scale bar = 5 μm). Lowering freezing temperature results in more porous ECM and thicker collagen fibers. (b) The number of viable (i.e., non-PI stained) cells decreases as the freezing temperature decreases (n=3). This is due to the increase in the number of both membrane-damaged (i.e., PI-stained) cells and detached cells. (c) More severe damage and breakage are observed in the cellular extensions when the freezing temperature decreases.
3.3 Effects of CPA addition on freezing-induced tissue deformation
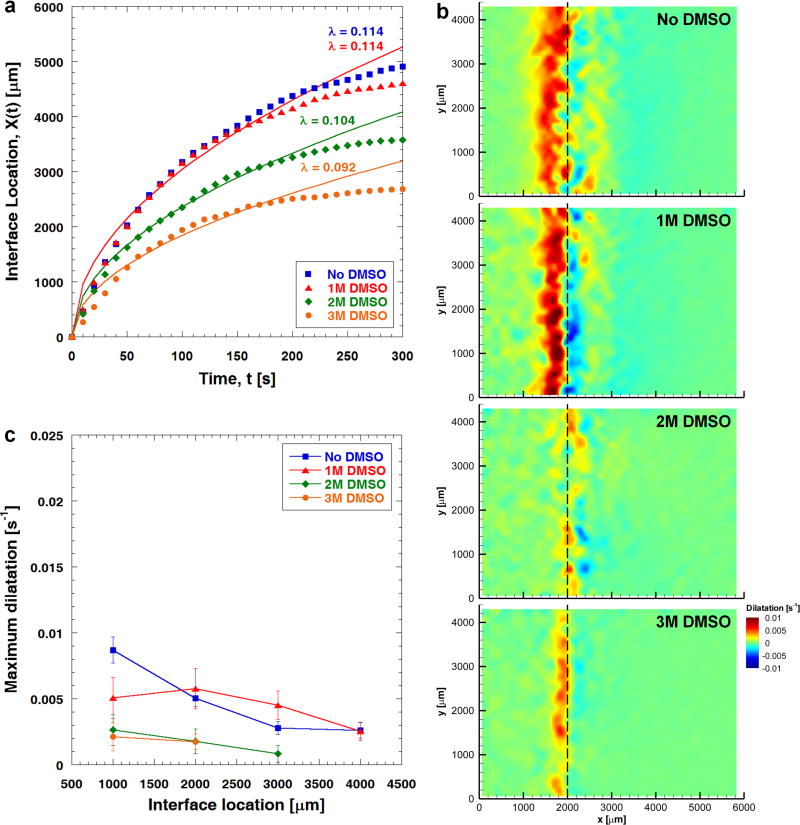
The freezing conditions undergone by the ETs differ when DMSO is added. As indicated in Fig. 4a, even though under the same freezing protocol (i.e., TL = −20 °C), the phase change interface stops at different times and locations, depending on the concentration of DMSO. The terminal interface location decreases with increasing concentration of DMSO. In addition, increasing DMSO concentration leads to a lower value of λ and slower freezing interface propagation. For 1 M DMSO-loaded ETs, λ is estimated to be 0.114 (R2 = 0.978), which is similar to that without DMSO. However, λ decreases to 0.104 (R2 = 0.987) and 0.092 (R2 = 0.974) for 2 M and 3 M DMSO-treated ETs, respectively.
Fig. 4.
Effects of DMSO treatment on freezing-induced tissue deformation when freezing temperature is set at −20 °C (n=3). (a) When the DMSO concentration increases, the terminal freezing interface location decreases, and the value of λ decreases, indicating a slower freezing interface. The terminal freezing interface is at approximately 4600, 3600, and 2600 μm when 1, 2, and 3 M DMSO, respectively, are used. (b) Local dilatations experienced by engineered tissues treated with different concentrations of DMSO when X(t) = 2000 μm. The local dilatation experienced by the ETs decreases significantly as the DMSO concentration increases from 1 M to 2 M. (c) The magnitude of the maximum dilatation experienced by engineered tissues treated with different concentrations of DMSO.
Fig. 4b and 4c illustrate the effects of DMSO treatment on the spatiotemporal deformation of ETs during freezing when TL = −20 °C. As seen in Fig. 4b, 1 M DMSO-treated ET deforms very similarly to that without DMSO treatment. However, the ET deforms significantly less as the concentration of DMSO increases to 2 M. When X(t) ≈ 2000 μm, the maximum local dilatation measured in the ETs loaded with 1 M DMSO is approximately 0.0057 ± 0.0015 s−1 (see Fig. 4c). This value reduces by 70% to 0.0017 ± 0.0005 s−1 when the concentration of DMSO increases from 1 M to 2 M. On the contrary, the increase from 2M to 3M does not cause a notable change of the deformation, and the dilatation of 3M DMSO-treated ETs is similar to or slightly lower than that of 2M DMSO-treated ETs (see Fig. 4c). Therefore, a DMSO threshold of 2M is observed when TL = −20 °C.
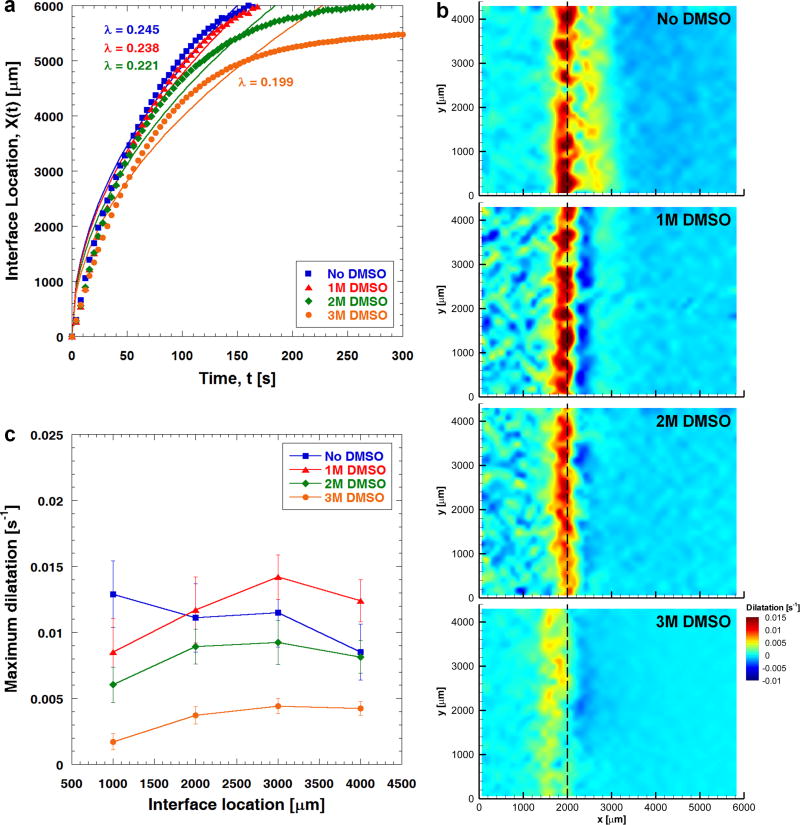
A similar threshold phenomenon is also observed when TL = −40 °C, as explained in Fig. 5. Here, Fig. 5a indicates that the freezing interface propagates faster as quantified by overall higher values of λ compared to those of the case TL = −20 °C. No significant change in the maximum local dilatation is observed over the range of 0 to 2 M DMSO (Figs. 5b and 5c). However, the maximum local dilatation decreases significantly to 0.0037 ± 0.0006 s−1 (X(t) ≈ 2000 μm) when the ETs are treated with 3 M DMSO, about 68% less than that measured for 1 M DMSO-treated ETs (0.0117 ± 0.0025 s−1). Interestingly, the threshold DMSO concentration increases as the freezing temperature decreases.
Fig. 5.
Effects of DMSO treatment on freezing-induced tissue deformation when freezing temperature is set at −40 °C (n=3). (a) Regardless of the DMSO concentration, the terminal freezing interface location remains at x = 6000 μm. However, the value of λ decreases with increasing DMSO concentration, implying a slower freezing interface. (b) Local dilatations experienced by engineered tissues treated with different concentrations of DMSO when X(t) = 2000 μm. The local dilatation experienced by the ETs decreases significantly when the DMSO concentration increases from 2 M to 3 M. (c) The magnitude of the maximum dilatation experienced by engineered tissues treated with different concentrations of DMSO.
3.4 Effects of CPA addition on ECM microstructure, cell viability and cytoskeletal structure
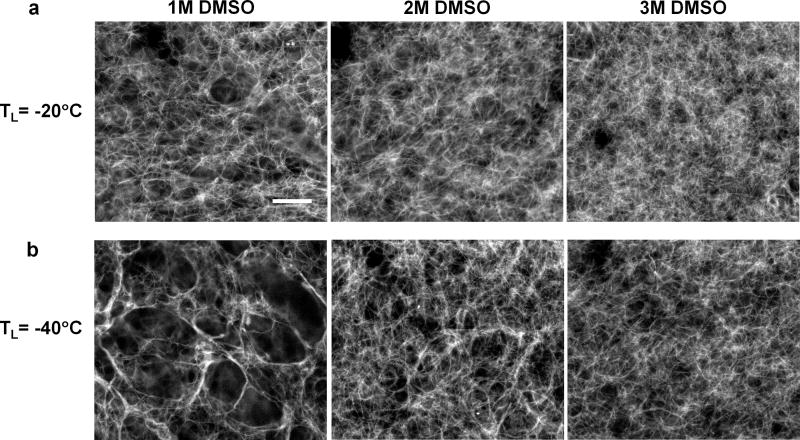
The effects of DMSO treatment (TL = −20 °C) on the post-thaw ECM microstructure are shown in Fig. 6a. The ECM microstructure of 1 M DMSO-treated ETs shows similar levels of damage to ETs frozen/thawed without DMSO (see Fig. 3a) including several large sparse voids and thicker collagen fibrils. With the addition of 2 M DMSO, the microstructure of the ECM seems to be better preserved so that no notable large voids and fine collagen fibrils are observed. The pore size decreases and approaches that of unfrozen ECM (compare to Fig. 3a) when the concentration of DMSO increases to 3 M. This reconfirms the existence of a threshold DMSO concentration to mitigate freezing-induced tissue deformation and to subsequently preserve the ECM microstructure for a given type of tissue. Similarly, for TL = −40 °C, increasing the concentration of DMSO from 2 M to 3 M also produced a dramatic improvement in maintaining the ECM microstructure after F/T (see Fig. 6b).
Fig. 6.
Effects of DMSO treatment on ECM microstructure for different freezing temperatures. (a) Scanning electron micrographs of frozen/thawed ECM microstructure for different concentrations of DMSO (scale bar = 5 μm) for TL = −20 °C. Significantly less alteration in ECM microstructure is observed when the DMSO concentration increases from 1 M to 2 M. (b) A similar threshold transition occurs for the freezing temperature of TL = −40 °C. The appearance of frozen/thawed ECM microstructure approaches that of unfrozen ECM when the DMSO concentration increases from 2 M to 3 M.
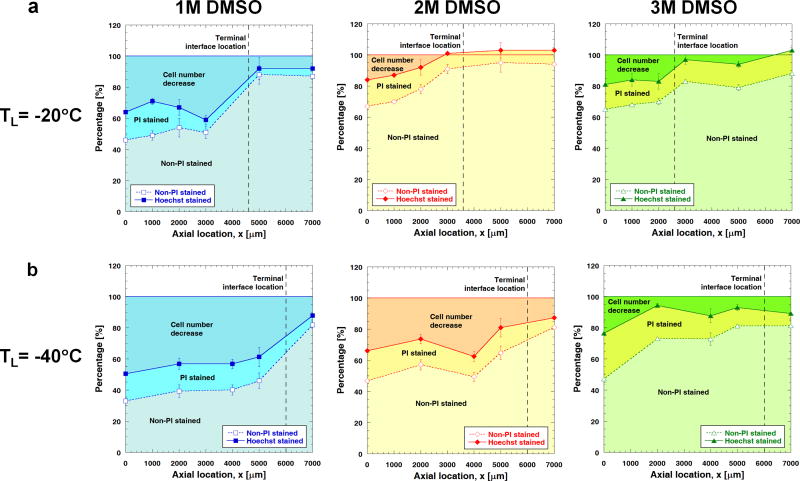
The effects of DMSO treatment on post-thaw cell viability at different axial locations in the ETs are shown in Fig. 7a (TL =−20 °C) and 6b (TL =−40 °C). The cell viability is improved with the use of DMSO. Interestingly, for each given freezing temperature, the number of PI-stained cells remains somewhat unchanged over the range of 1 M to 3 M DMSO. In contrast, the number of detached cells decreases significantly when the DMSO concentration increases from 1 M to 2 M for TL = −20 °C, and from 2 M to 3 M for TL = −40 °C. These results are consistent with the dilatation measured in the ETs, which decreases significantly in magnitude as a result of increasing DMSO concentration above a certain threshold for a given freezing temperature. The freezing-induced mechanical insults, instead of thermal, appear to be reduced significantly as a result of using DMSO, whose effective concentration seems to be dependent on freezing temperature.
Fig. 7.
Effects of DMSO treatment on cell viability for different freezing temperatures (n=3 for all cases). (a) When TL = −20 °C, the number of attached viable (i.e., non-PI stained) cells increases significantly over the range of 1 M to 2 M DMSO. This increase in the number of viable cells is due to the decrease in the number of detached cells, given that the number of attached damaged (i.e., PI stained) cells remains almost the same over the range of 1 M to 3 M DMSO. (b) For TL = −40 °C, when the DMSO concentration increases from 2 M to 3 M, the number of attached viable cells increases significantly. This increase is due to a smaller number of detached cells as the number of attached necrotic cells remains unchanged from 2 M to 3 M DMSO.
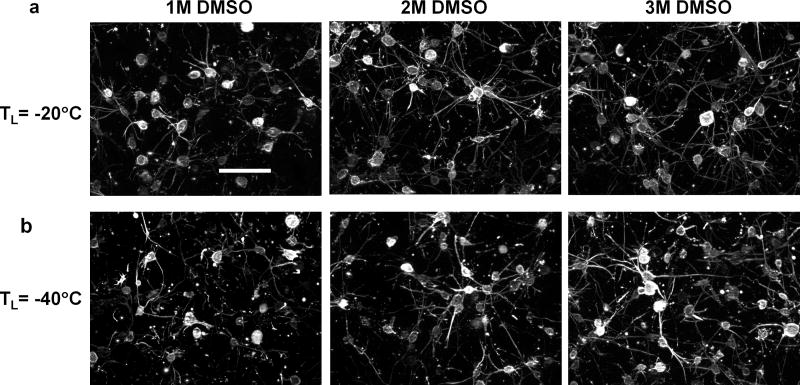
The effects of different DMSO concentrations on the cell-matrix adhesions are summarized in Figs. 8a and 8b. By using a higher concentration of DMSO, the dendritic extensions as well as the cell-matrix adhesions are better preserved. The morphology of the fibroblasts becomes very similar to that of the unfrozen case when the ETs are treated with 2 M DMSO for TL = −20 °C, and 3 M DMSO for TL = −40 °C.
Fig. 8.
Effects of DMSO treatment on actin cytoskeletal structure for different freezing temperatures. (a) For TL = −20 °C, the cellular extensions are better preserved after F/T when the DMSO concentration increases from 1 M to 2 M. (b) For TL = −40 °C, the cellular extensions and cell-matrix adhesion appear to be intact and similar to those of unfrozen ECM after F/T when the DMSO concentration increases from 2 M to 3 M.
3.5 Thermophysical analysis of freezing-induced volumetric expansion
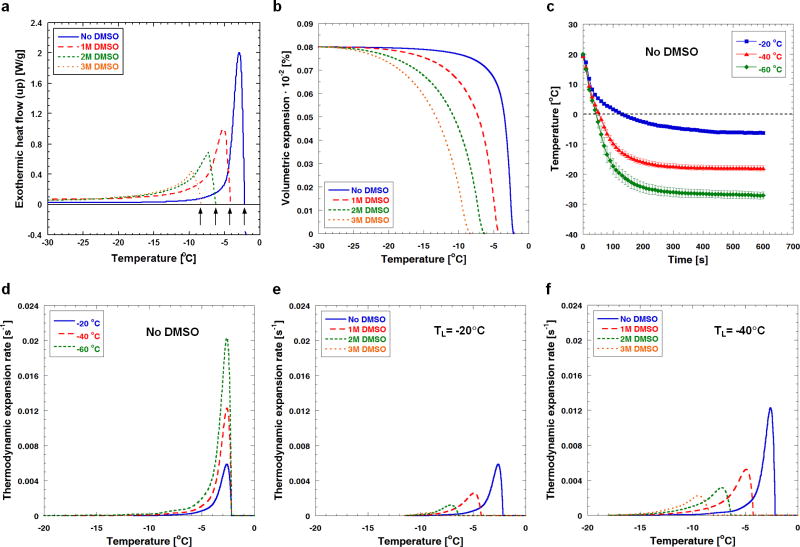
Typical DSC thermograms of ETs without and with DMSO treatment are shown in Fig. 9a. As the DMSO concentration increases, the water/ice phase change temperature (noted with arrows) decreases, and the magnitude of the latent heat (i.e., area under each thermogram) also decreases. As shown in Fig. 9b, as the temperature decreases, the volumetric expansion increases and ultimately reaches approximately 8%, which implies that all available interstitial water changes to ice. Without DMSO treatment, most of the volumetric expansion occurs near the phase change temperature, but the addition of DMSO spreads out the expansion over a broader temperature range. These volumetric expansions have been further analyzed to estimate the thermodynamic expansion rates using temperature histories measured at x = 2 mm (Fig. 9c), and are plotted against temperature for all experimental groups in Figs. 9d, 9e and 9f. Regardless of DMSO concentration, the maximum thermodynamic expansion rate occurs at the phase change temperature, and increases as the freezing temperature decreases. Without DMSO, the maximum thermodynamic expansion rate is calculated to be approximately 0.0058 s−1 for TL = −20 °C, and the value increases to 0.0202 s−1 as the freezing temperature decreases to −60 °C (i.e., approximately 250% increase). When DMSO is used, the maximum thermodynamic expansion rate decreases with increasing DMSO concentration. For TL = −20 °C, the maximum thermodynamic expansion rate calculated for the ETs loaded with 1 M DMSO is 0.0026 s−1. This value reduces to about 0.0003 s−1 when the DMSO concentration increases to 3 M. These results suggest that lowering the freezing temperature (i.e., rapid freezing) significantly increases the freezing-induced swelling rates (compare Figs. 9e and 9f), but the addition of DMSO reduces the swelling rates, and thus post-thaw ECM microstructural changes remain minimal.
Fig. 9.
Thermodynamic freezing response of engineered tissues as a function of temperature determined using DSC technique. (a) Exothermic heat flow thermograms of engineered tissues treated with different concentrations of DMSO. As indicated by the freezing peaks, the water/ice phase change temperature decreases with increasing DMSO concentration. (b) Volumetric expansion occurring in engineered tissues during freezing. Volumetric expansion generally decreases with increasing DMSO concentration. (c) Local temperature profiles during freezing of engineered tissues without DMSO. The local temperature at axial distance x = 2 mm from the edge of the low-temperature reservoir is measured (n = 3). Generally, a higher cooling rate and a lower steady-state temperature are observed at the given axial location when the freezing temperature decreases. (d) Thermodynamic expansion rates experienced by engineered tissues for different freezing temperatures without DMSO. Thermodynamic expansion rate increases with lowering temperature. (e) For TL = −20 °C, as more DMSO is added the thermodynamic expansion rate decreases (f) For TL = −40 °C the same observation prevails.
4. Discussion
4.1 Cryopreservation parameters can control ECM microstructure
Cryopreservation can cause tissue- level microstructural change besides cell-level injury. Muldrew et al. (Muldrew et al. 2000) have reported that the collagenous ECM of cartilage becomes more porous and open after freezing and thawing (F/T), and this is claimed to be due to polycrystalline ice formation and its subsequent mechanical disruption of the matrix architecture. It has been observed that conventional cryopreservation of heart valves causes significant deterioration of their matrix structures (Brockbank et al. 2000; Schenke-Layland et al. 2006; Schenke-Layland et al. 2007). In addition, collagen matrices have been shown to undergo structural changes due to freezing such as enlarged pore structures and increased collagen fibril diameters (Han et al. 2009). CPA is proved to be an important parameter to protect ET microstructure. Several protective mechanisms of CPAs have been proposed at the cellular level, but tissue-level protection mechanisms are not well understood (Mazur 1984; Karlsson and Toner 1996; Pegg 2002; Han and Bischof 2004). Schenke-Layland et al. (Schenke-Layland et al. 2007) have demonstrated that cryopreservation in the presence of 3 M DMSO led to less ECM damage within cardiac tissues than conventional cryopreservation without the CPA. Neidert et al. (Neidert et al. 2004) reported that cryopreserved collagenous tissue equivalents in the presence of 0.5 M DMSO were found to retain their opacity and ECM structure as well as cellular viability and mechanical properties. Still the mechanism of freezing and addition of CPA on ECM microstructural change is still unknown.
4.2 Tissue deformation and cell damage caused by fluid-matrix interaction
The present results show that lowering freezing temperature causes larger tissue dilatation (i.e., expansion rate) during freezing (Figs. 2b and 2c) because of more involved fluid-matrix interaction and severed ECM microstructure (Fig. 3a). This might be caused by the increased local cooling rate which does not provide enough time for the interstitial fluid to propagate through the matrix. As the freezing temperature decreases, the location of the maximum dilatation becomes closer to the freezing interface and moves into the unfrozen region (Fig. 2b). This might be a consequence of the local tissue swelling as the excess interstitial fluid is transported from the freezing interface into the unfrozen region.
Lowering freezing temperature results in lower cell viability and damaged actin cytoskeleton structure as shown in Figs. 3b and 3c, respectively. In addition, the number of detached cells increases with lowering freezing temperature. This could be explained by the freezing-induced deformation of the ETs. As reported in Figs. 2b and 3a, larger tissue deformation and weaker ECM structure is observed when the freezing temperature decreases. This implies that the cells in the ETs, when frozen to a lower temperature, experience greater tensional strain as well as cryo-insults, resulting in more cells detaching from the ECM.
4.3 Thermo mechanical explanation of improved tissue preservation by DMSO addition
DMSO reduces the local deformation experienced by the tissues during freezing (Figs. 4a and 4b, 5a and 5b), and in this work the cryoprotective effect of DMSO is shown to be threshold-dependent. 2 M and 3 M concentrations have been shown to be the effective levels of DMSO for better preserving the functionalities of ETs when the freezing temperature was set at −20 and −40 °C, respectively. From these results it is possible to quantify an approximate maximum limit of tissue deformation to achieve an acceptable ECM microstructure. For this particular tissue system, this value is approximately e = 0.002 s−1 (Figs. 4b and 5b, 9e and 9f). These results imply that the threshold concentrations are affected by both tissue type and freezing protocol. The existence of threshold concentrations of CPA can be explained as follows. Addition of CPA causes the phase temperature to decrease (Fig. 9a), a smaller amount of ice formation (i.e., smaller expansion of tissue), broader mushy region and a larger unfrozen portion. Thermally, these changes result in lower thermal diffusivity in the mushy region (because of lower thermal conductivity and higher specific heat) even with smaller latent heat. This allows more time for the interstitial fluid to propagate through the matrix and consequently results in reduced expansion in the frozen region. Thus, below the DMSO threshold the resulting deformation magnitude decreases. Adding more DMSO than this does not provide any further cryoprotection.
4.4 Threshold feature of CPA in preserving cells and ECM
The existence of a CPA threshold is clear from the post-thaw ECM microstructure, cell viability and cell detachment results. Results confirm that decreased tissue deformation during freezing using CPA could lead to a less damaged post-thaw ECM microstructure as shown in Fig. 6a and 5b of this study, as well as in other previous studies (Neidert et al. 2004; Schenke-Layland et al. 2007). The same is applicable for the number of attached live cells. For the freezing temperature of −20 °C, 2 M DMSO yields the highest cell survival and best ECM microstructure, and no beneficial effect is observed beyond 2 M. Similarly, for the freezing temperature of −40 °C, no significant improvement in the number of live cells and acceptable ECM microstructure was observed until 3 M DMSO is used. As expected, the number of attached live cells (i.e., Hoechst stained) in the frozen region increased as a result of using CPA (see Figs. 7a and 7b). Interestingly, the number of cells experiencing membrane damage (i.e., PI stained) decreases when DMSO is introduced at the concentration of 1 M, but it remains almost the same thereafter over the range of 1 M to 3 M. The initial improved cell viability is due to the reduced cellular cryoinjury during cryopreservation with the CPA. At the same time, the number of detached cells decreases significantly as a result of using the CPA. This implies that freezing-induced tissue deformation could be the dominant mechanism through which cells are damaged during tissue cryopreservation.
4.5 Relation between thermodynamic and mechanical deformation of ETs
Tissue deformation is a consequence of fluid-matrix interaction quantified here as dilatation of the solid matrix. The dilatation, e, measured during freezing of the ETs is proportional to the thermodynamic expansion rate, dV/dt, computed from the DSC thermograms:
| (6) |
where the second term (i.e., due to interstitial fluid transport) is known to be a function of hydraulic permeability and elastic modulus (Han et al. 2009). The fluid velocity v⃗f and the solid matrix displacement vector U⃗ are related using the continuity equation as follows,
| (7) |
From this equation it should be noted that the fluid velocity also proportionately increases with increasing deformation of the matrix. This causes higher flow at the interface from the frozen to the unfrozen region. This high fluid flux may render a more severe impact on the solid matrix. The dilatation measured in the tissue deformation experiments generally agrees well with the thermodynamic expansion rate computed; the order of magnitude for the maximum thermodynamic expansion rates was similar to that for the maximum dilatation measured. The thermodynamic expansion rate (due to water/ice phase change) was always higher than the dilatation observed in the frozen region when CPA was absent. This might be explained by the fact that interstitial fluid tended to move away from the freezing interface/frozen region into the unfrozen region.
4.6 Preservation of cytoskeletal structure by CPA addition
The local expansion and compression of the ETs during freezing may inflict significant strains on the dendritic extensions of the cells (i.e., sites of cell-matrix adhesion), causing the cells to detach from their surrounding matrix. In addition, extracellular ice formation mechanically constrains and deforms the cells (Ishiguro and Horimizu 2008). Mechanical constraint imposed by extracellular and intracellular ice could cause cell deformation, ultimately leading to cell detachment from the surrounding matrix. Cell-cell and cell-matrix junctions have also been shown to be potential targets of injury during tissue cryopreservation. Experimental evidence has suggested that intercellular junctions and cellular adhesion sites are affected by exposure to freezing conditions (Armitage et al. 1994; Liu and McGrath 2006). The aforementioned notions are supported by the present results (Fig. 3c), which clearly show the physical damage to the cellular actin cytoskeleton after F/T without CPA. It is also observed in the current study that the actin cytoskeleton is better maintained when CPA is present during tissue cryopreservation. CPA increases the unfrozen fraction by suppressing ice formation, and protects cells from mechanical stresses and damage caused by extracellular and intracellular ice formation. CPA also increases the fluidity of the cell membrane (Notman et al. 2006), allowing the cell membrane to sustain external strains to a certain extent without being permanently damaged.
4.7 Broader implications and future studies
The cooling rate in the ET studied varies in a spatiotemporal fashion for different cooling temperatures as discussed earlier. It can be seen that at x = 0 mm, the cooling rate is between 0.69°C/ min to 42.5°C/ min. This is a typical range of cooling rates during freezing tissue samples. For example, liver tissue was exposed from 5 °C/min slow cooling to 50 °C/min slam cooling (Pazhayannur and Bischof, 1997). Therefore, in this study, the cooling rates applied overlap with the cooling rates used in cryopreservation practice and makes this study relevant for tissue cryopreservation.
The results from this study can have broader impact in cryopreservation of other types of cells and tissues. Although fibroblast in collagen was used as a model system in the present study, the underlying interaction mechanisms among cells, ECM and fluid are applicable to other tissue types. Thus, the values of the threshold strain for preserved tissue microstructure are cell and tissue type dependent, but the existence of the threshold would be generic. Further studies are required to know the corresponding threshold strain in other tissues. The proposed strategy to use the amount of CPA and freezing temperature to keep the dilatation below the threshold level can be applicable to other types of tissues, and it can also minimize the toxicity of CPAs.
It is also worth investigating the effects of cell configurations in tissue (i.e., cell monolayer, cells sandwiched between collagen gel and cells embedded in collagen matrix) on the strain threshold value. Depending on the cytoskeletal structure of cells within the matrix, their dilatation patterns and post-thaw viability are thought to be substantially changed. Although the underlying interaction mechanism remains the same, the role of cytoskeletal structure is anticipated to become important.
5. Conclusion
In summary, the freezing-induced cell-fluid-matrix interactions are considered to be the key mechanisms that dictate the outcomes of tissue cryopreservation. These interactions, as shown in the present study, can be manipulated by changing two fundamental parameters of cryopreservation protocols – freezing temperature and CPA concentration. It was revealed that the tissue deformation increases with lowering freezing temperature. However, the acceptable level of post-thaw tissue microstructure and cell viability is limited by tissue specific deformation, as concluded from ET dilation, SEM and immunofluorescence images. Addition of CPA can alleviate the freezing-induced ET deformation, but a freezing temperature-dependent threshold CPA concentration exists. Beyond this concentration further addition of CPA does not help to improve the tissue microstructure and cell viability. Both the thermodynamic and mechanical components of tissue deformation are considered to explain the freezing-induced CFM interactions. Mechanistic relationships between the cryopreservation parameters and the resulting cell-fluid-matrix interactions are proposed to design effective cryopreservation protocols for various tissue systems.
Supplementary Material
Highlights.
Tissue cryopreservation adversely affects ECM microstructure and cell viability.
Freezing induced tissue deformation should be kept below a tissue specific limit.
A freezing temperature dependent threshold cryoprotectant concentration exists.
Thermomechanical tissue deformation was characterized using cell deformetry and DSC.
Freezing parameters were correlated to viability, cytoskeletal and ECM structure.
Acknowledgments
This research was supported by grants from the National Institutes of Health/National Institute of Biomedical Imaging and Bioengineering, R01 EB008388, and the National Science Foundation, CBET-1009465. The scanning electron microscope images were obtained at the Life Science Microscopy Facility at Purdue University.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aggarwal SJ, Baxter CR, Diller KR. Cryopreservation of skin: an assessment of current clinical applicability. Journal of Burn Care & Rehabilitation. 1985;6:469–476. doi: 10.1097/00004630-198511000-00003. [DOI] [PubMed] [Google Scholar]
- Armitage WJ, Juss BK, Easty DL. Response of epithelial (MDCK) cell junctions to calcium removal and osmotic stress is influenced by temperature. Cryobiology. 1994;31:453–460. doi: 10.1006/cryo.1994.1055. [DOI] [PubMed] [Google Scholar]
- Brockbank KGM, Lightfoot FG, Song YC, Taylor MJ. Interstitial ice formation in cryopreserved homografts: A possible cause of tissue deterioration and calcification in vivo. Journal of Heart Valve Disease. 2000;9:200–206. [PubMed] [Google Scholar]
- David P, Alexandre E, Audet M, Chenard-Neu MP, Wolf P, Jaeck D, Azimzadeh A, Richert L. Engraftment and albumin production of intrasplenically transplanted rat hepatocytes (Sprague-Dawley), freshly isolated versus cryopreserved, into Nagase analbuminemic rats (NAR) Cell Transplant. 2001;10:67–80. [PubMed] [Google Scholar]
- Devireddy RV, Neidert MR, Bischof JC, Tranquillo RT. Cryopreservation of collagen-based tissue equivalents I: Effect of freezing in the absence of cryoprotective agents. Tissue Engineering. 2003;9:1089–1100. doi: 10.1089/10763270360728008. [DOI] [PubMed] [Google Scholar]
- Elder E, Chen Z, Ensley A, Nerem RM, Brockbank KGM, Song YS. Enhanced tissue strength in cryopreserved, collagen-based blood vessel constructs. Transplantation Proceedings. 2005;37:4625–4629. doi: 10.1016/j.transproceed.2005.10.033. [DOI] [PubMed] [Google Scholar]
- Han B, Bischof JC. Engineering challenges in tissue preservation. Cell Preservation Technology. 2004;2:91–112. [Google Scholar]
- Han B, Grassl E, Barocas V, Coad J, Bischof JC. A cryoinjury modeling using engineered tissue equivalents for cryosurgical applications. Annals of Biomedical Engineering. 2005;33:980–990. doi: 10.1007/s10439-005-3478-z. [DOI] [PubMed] [Google Scholar]
- Han B, Miller JD, Jung JK. Freezing-induced fluid-matrix interaction in poroelastic material. Journal of Biomechanical Engineering. 2009;131:021002. doi: 10.1115/1.3005170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishiguro H, Horimizu T. Three-dimensional microscopic freezing and thawing behavior of biological tissues revealed by real-time imaging using confocal laser scanning microscopy. International Journal of Heat and Mass Transfer. 2008;51:5642–5649. [Google Scholar]
- Ishine N, Rubinsky B, Lee CY. Transplantation of mammalian livers following freezing: vascular damage and functional recovery. Cryobiology. 2000;40:84–89. doi: 10.1006/cryo.1999.2225. [DOI] [PubMed] [Google Scholar]
- Karlsson JOM, Cravalho E, Rinkes IHMB, Tompkins RG, Yarmush ML, Toner M. Nucleation and growth of ice crystals inside cultured hepatocytes during freezing in the presence of Dimethyl Sulfoxide. Biophysical Journal. 1993;65:2524–2536. doi: 10.1016/S0006-3495(93)81319-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson JOM, Toner M. Long-term storage of tissue by cryopreservation: critical issues. Biomaterials. 1996;17:243–256. doi: 10.1016/0142-9612(96)85562-1. [DOI] [PubMed] [Google Scholar]
- Kubo K, Kuroyanagi Y. The possibility of long-term cryopreservation of cultured dermal substitute. Artificial Organs. 2005;29:800–805. doi: 10.1111/j.1525-1594.2005.00132.x. [DOI] [PubMed] [Google Scholar]
- Liu B, McGrath JJ. Effects of two-step freezing on the ultra-structural components of murine osteoblast cultures. CryoLetters. 2006;27:369–374. [PubMed] [Google Scholar]
- Mansbridge J. Commercial considerations in tissue engineering. Journal of Anatomy. 2006;209:527–532. doi: 10.1111/j.1469-7580.2006.00631.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazur P. Freezing of living cells: mechanisms and implications. American Journal of Physiology. 1984;247:C125–142. doi: 10.1152/ajpcell.1984.247.3.C125. [DOI] [PubMed] [Google Scholar]
- Muldrew K, Novak K, Yang H, Zernicke R, Schacher NS, McGann LE. Cryobiology of articular cartilage: Ice morphology and recovery of chondrocytes. Cryobiology. 2000;40:102–109. doi: 10.1006/cryo.2000.2236. [DOI] [PubMed] [Google Scholar]
- Neidert MR, Devireddy RV, Tranquillo RT, Bischof JC. Cryopreservation of collagen-based tissue equivalents II: Improved freezing in the presence of cryoprotective agents. Tissue Engineering. 2004;10:23–32. doi: 10.1089/107632704322791664. [DOI] [PubMed] [Google Scholar]
- Nerem RM. Tissue engineering: the hope, the hype, and the future. Tissue Engineering. 2006;12:1143–1150. doi: 10.1089/ten.2006.12.1143. [DOI] [PubMed] [Google Scholar]
- Nerem RM. Regenerative medicine: the emergence of an industry. Journal of the Royal Society Interface. 2010;7:S771–775. doi: 10.1098/rsif.2010.0348.focus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notman R, Noro M, O’Malley B, Anwar J. Molecular basis for dimethlysulfoxide (DMSO) action on lipid membranes. Journal of American Chemical Society. 2006;128:13982–13983. doi: 10.1021/ja063363t. [DOI] [PubMed] [Google Scholar]
- Pazhayannur PB, Bischof JC. Measurement and simulation of water transport during freezing in mammalian liver tissue. Journal of Biomechanical Engineering. 1997;119:269–277. doi: 10.1115/1.2796091. [DOI] [PubMed] [Google Scholar]
- Pegg DE. The history and principles of cryopreservation. Seminars in Reproductive Medicine. 2002;20:5–13. doi: 10.1055/s-2002-23515. [DOI] [PubMed] [Google Scholar]
- Schenke-Layland K, Madershahian N, Riemann I, Starcher B, Halbhuber KJ, Konig K, Stock UA. Impact of cryopreservation on extracellular matrix structures of heart valve leaflets. The Annals of Thoracic Surgery. 2006;81:918–926. doi: 10.1016/j.athoracsur.2005.09.016. [DOI] [PubMed] [Google Scholar]
- Schenke-Layland K, Xie J, Heydarkhan-Hagvail S, Hamm-Alvarez SF, Stock UA, Brockbank KG, MacLellan WR. Optimized preservation of extracellular matrix in cardiac tissues: implication for long-term graft durability. Ann Thorac Surg. 2007;83:1641–1650. doi: 10.1016/j.athoracsur.2006.12.005. [DOI] [PubMed] [Google Scholar]
- Song YC, Hunt CJ, Pegg DE. Cryopreservation of the common carotid artery of the rabbit. Cryobiology. 1994;31:317–329. doi: 10.1006/cryo.1994.1038. [DOI] [PubMed] [Google Scholar]
- Teo KY, DeHoyos TO, Dutton JC, Grinnell F, Han B. Effects of freezing-induced cell-fluid-matrix interactions on the cells and extracellular matrix of engineered tissues. Biomaterials. 2011;32:5380–5390. doi: 10.1016/j.biomaterials.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teo KY, Dutton JC, Han B. Spatiotemporal measurement of freezing-induced deformation of engineered tissues. Journal of Biomechanical Engineering. 2010;132:031003. doi: 10.1115/1.4000875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Pegg DE, Lorrison J, Vaughan D, Rooney P. Further work on the cryopreservation of articular cartilage with particular reference to the liquidus tracking (LT) method. Cryobiology. 2007;55:138–147. doi: 10.1016/j.cryobiol.2007.06.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.