FIGURE 2.
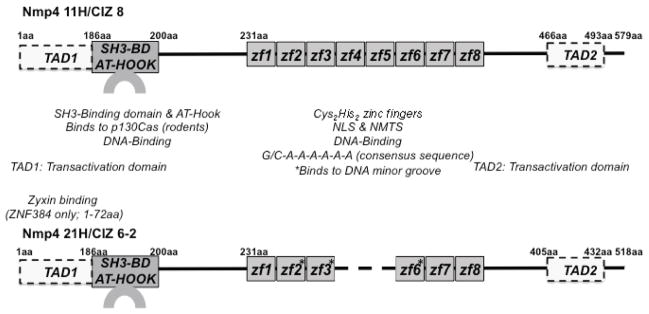
Nmp4/CIZ has the functional domains of a transcription factor and signaling molecule. Rat isoforms Nmp4 11H [CIZ 8] and Nmp4 21H [CIZ 6-2] are shown.28,55 The amino acid positions are indicated by numerals but are not positioned to scale. The Cys2His2 zinc fingers act as a nuclear localization signal (NLS) and a nuclear matrix-targeting signal (NMTS); for Nmp4 11H, the zinc fingers #4–#8 are minimally required for the NLS and NMTS.76 The Nmp4/CIZ SH3-binding domain (SH3-BD) associates with p130Cas in rodent cells; however, the absence of a single proline residue in the human ortholog ZNF384 obviates p130Cas association, but instead ZNF384 binds to zyxin.28,79 The transactivation and DNA-binding domains were mapped using Nmp4 21H.75 DNA binding to the minor groove of DNA is mediated by the zinc fingers #2, #3, and #6 (asterisks), whereas the AT-hook motif, which overlaps with the p130Cas SH3-binding sequence, weakly associates with the DNA major groove. There are context-dependent transactivation domains within the first 186aa (TAD1) of the protein and within a polyglutamine/alanine repeat (TAD2) (see Ref. 75 for full discussion).
