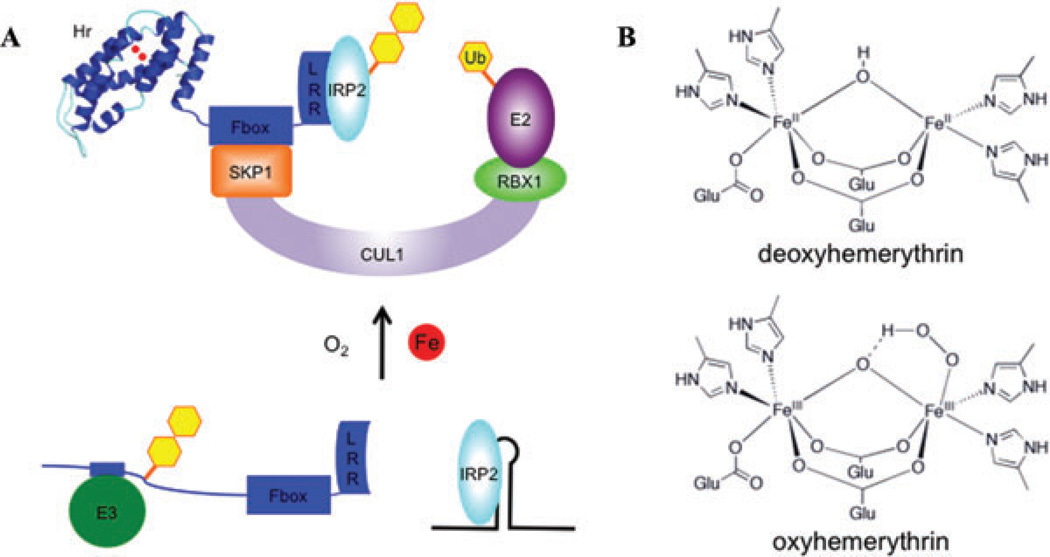
Figure 3.
Model of FBXL5’s proposed role in cellular iron homeostasis. (A) In states of low iron bioavailability, FBXL5’s hemerythrin domain is likely destabilized, allowing for a putative E3 ubiquitin ligase to recognize a degron and promote FBXL5’s degradation. Limiting levels of FBXL5 result in IRP2 stabilization and binding to IREs. In contrast, when iron bioavailability increases, FBXL5’s hemerythrin domain forms a stable alpha helical bundle by directly binding iron and oxygen. In this state, FBXL5 is able to mask its degron and assemble into a SCF complex that promotes the polyubiquitination and degradation of IRP2. (B) Model of hemerythrin iron- and oxygen-binding site. Imidazole and carboxylate residues from conserved amino acid side chains bind a diiron center connected by a bridging oxygen atom (μ-oxo). Oxygen can be reversibly bound onto the penta-coordinate iron and forms a peroxo radical species that is stabilized by protonation and coordination to the bridging oxygen atom.