Fig 4.
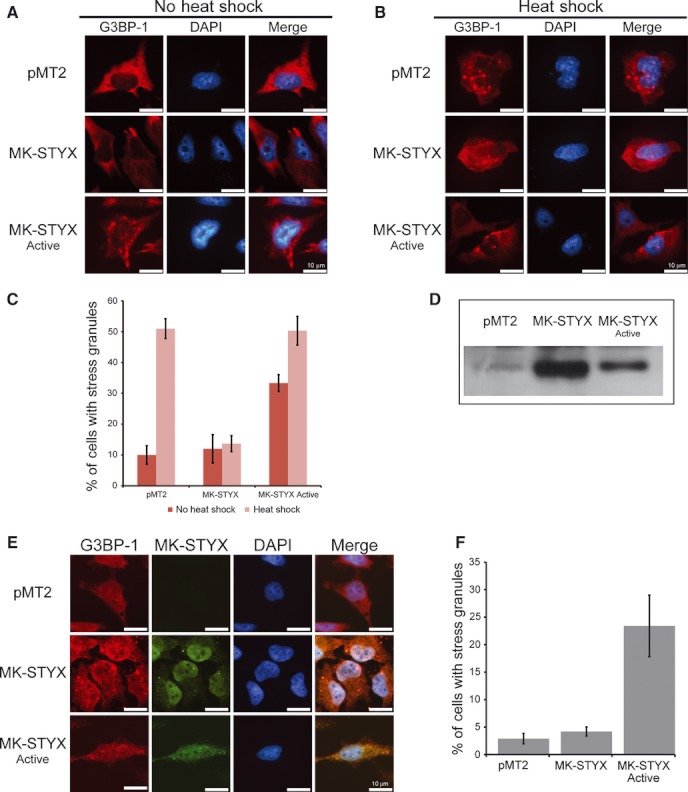
Inhibition of heat shock-induced stress granule formation by MK-STYX Representative examples of the subcellular distribution of endogenous G3BP-1 without heat shock (A) or after heat shock at 41 °C (B), in the presence of pMT2 (empty vector), MK-STYX or the active mutant as indicated. Cells transfected with MK-STYX did not form heat shock-induced granules, whereas the active mutant induced granules in the presence or absence of heat shock. Cells were fixed, stained with anti-G3BP and Cy3-conjugated goat anti-mouse sera and DAPI, and analyzed 24 h post-transfection for heat shock-induced stress granule assembly by fluorescence microscopy. Merged images show the localization of endogenous G3BP-1 (red) relative to DAPI-stained nuclei (blue). Scale bar = 10 μm. (C) Cells were scored for the presence or absence of stress granules. Three replicate experiments were performed (n = 100 cells for each experiment); error bars indicate the SEM. (D) Blots were probed with anti-FLAG to visualize the presence of MK-STYX or active mutant. (E) Representative examples of the subcellular distribution of endogenous G3BP-1 and MK-STYX or MK-STYXactive. Cells were fixed, stained with anti-G3BP and Cy3-conjugated goat anti-mouse sera, FLAG conjugated to FITC (anti-FLAG-FITC) for the detection of FLAG-tagged MK-STYX or MK-STYXactive and DAPI. (F) Cells overexpressing MK-STYX or MK-STYXactive (green) were scored for the presence or absence of stress granules. Merged images show the localization of endogenous G3BP-1 (red) relative to MK-STYX (green), MK-STYXactive (green) or DAPI-stained nuclei (blue). Scale bar = 10 μm.
