Fig 5.
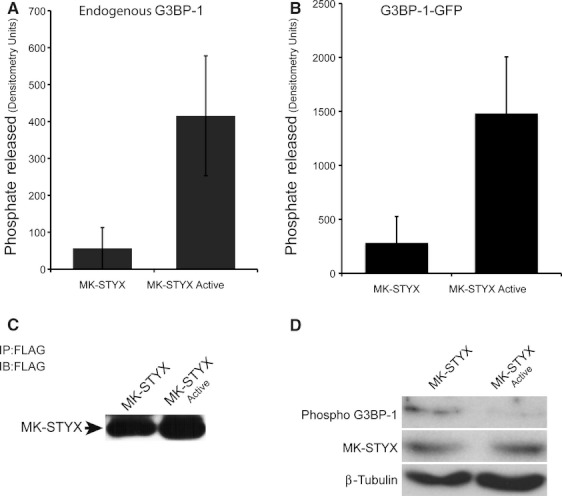
MK-STYX active mutant dephosphorylates G3BP-1. HeLa cells or HeLa cells expressing G3BP-1-GFP were labelled with [γ-32P]ATP, lysed and immunoprecipitated with anti-G3BP to obtain 32P-G3BP as substrate for a phosphatase liquid assay. Wild-type pMT2-FLAG-MK-STYX-FLAG and active mutant MK-STYX were expressed in HeLa cells, immunoprecipitated with anti-FLAG and assayed for phosphatase activity against (A) 32P-G3BP or (B) 32P-G3BP-GFP immunoprecipitates as substrate. The active mutant dephosphorylated endogenous G3BP-1, as well as G3BP-1-GFP. Three replicate experiments were performed. Error bars indicate the SEM. (C) An aliquot of the anti-FLAG immunoprecipitate (IP) used in the assays was resolved by SDS/PAGE and analyzed. Blots (IB) were probed with anti-FLAG to visualize immunoprecipitated MK-STYX, confirming the presence of wild-type and active mutant MK-STYX in the phosphatase assays. (D) HeLa cells expressing MK-STYX or MK-STYXactive were lysed and resolved by SDS/PAGE and analyzed. Blots were probed with anti-phosphoG3BP149 to determine the phosphorylation status of G3BP-1 at Ser149, anti-FLAG to confirm the presence of MK-STYX and β-tubulin as a loading control.
