Table 1.
MannichMCR between pyrrole, paraformaldehyde and aniline under various conditionsa
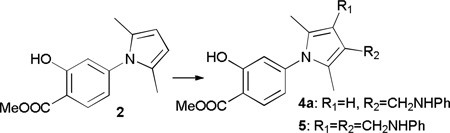 | |||
|---|---|---|---|
| Entry | Catalyst | Ratio of 4a/5/2 %b |
Isolated yield % |
| 1 | HOAc (20 mol%) | 52//22/26 | |
| 2 | TFA (20 mol%) | NA | |
| 3 | Proline (20 mol%) | 7/0/93 | |
| 4 | PTSA (20 mol%) | 21/5/74 | |
| 5 | PhCO2H(20 mol%) | 28/13/59 | |
| 6 | 2 M HCl (20 mol%) | 26/4/70 | |
| 7 | MeOCH2CO2H (20 mol%) | 45/36/19 | |
| 8 | (3,5-(CF3)2-PhNH)2CS (20 mol%) | no reaction | |
| 9 | HOAc (100 mol%) | 59//17/24 | |
| 10c | HOAc (100 mol%) | 45/9/46 | 85 (4a)e |
| 11d | MeOCH2CO2H (100 mol%) | 0/100/0 | 75 (5) |
the reaction was carried out at rt for 24 h in 1 mL CH2Cl2 with 2 (0.1mmol), paraformaldehyde (0.12mmol), aniline (0.12mmol);
the ratio is based on UV absorption in LC-MS studies of crude reaction mixture.
2 equiv. of pyrrole, 2 equiv. of paraformaldehyde, and 1 equiv. of aniline, were used.
1 equiv. of pyrrole, 3 equiv. of formaldehyde, and 3 equiv. of aniline were used, reaction time was 48 h.
based on 1 equiv. aniline
