Table 2.
Tropane synthesis via azomethine
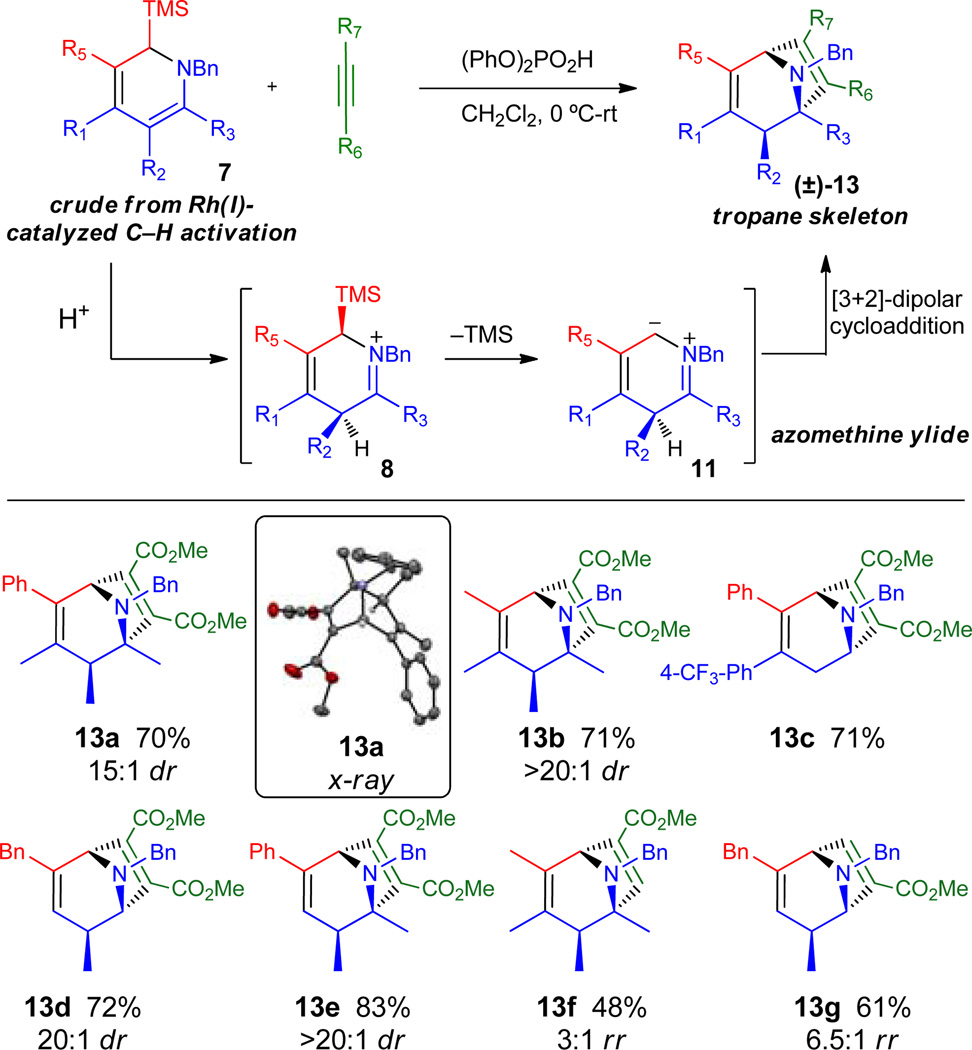 |
Yields correspond to isolated product after silica gel chromatography and represent overall yields from the imine precursor 6. Diastereoselectivity was determined by 1H NMR of the crude reaction mixture.
Conducted at −78 °C in toluene.
