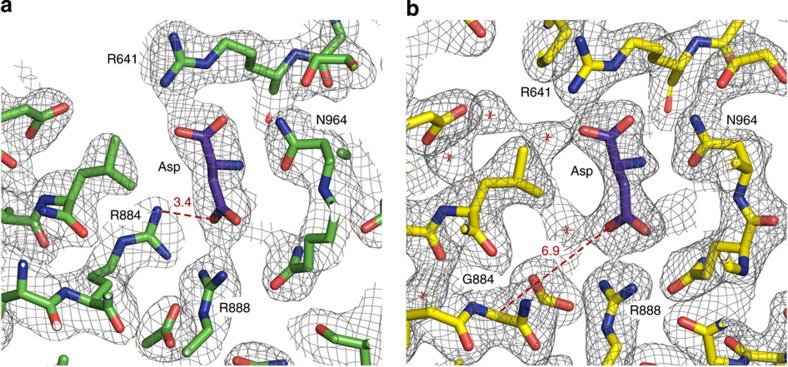
Figure 2. Comparison of the inhibitor-binding sites.
(a) C3 PEPC from F. pringlei. (b) C4 PEPC from F. trinervia. Arg884 in C3 PEPC functions as a clamp by providing an additional hydrogen bond for inhibitor binding, while Gly884 in C4 PEPC is more than 6 Å away from the inhibitor. Structures and 2Fo−Fc electron densities at 1.2σ of the malate/aspartate-binding pockets.