Figure 7.
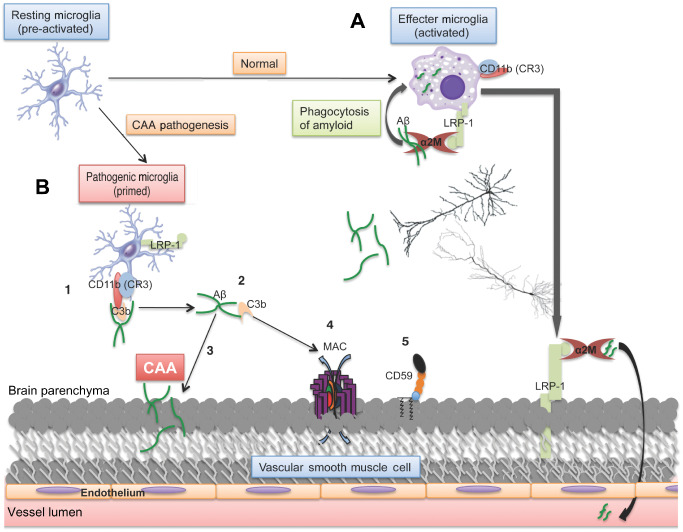
Proposed mechanism of MAC and Aβ deposition in CAA pathogenesis. A. In the healthy brain, activated microglia become amoeboid and clear Aβ through phagocytosis via α2M and LRP‐1. B. In AD with CAA, LRP‐1 and α2M are both decreased in human tissue, resulting in the compensatory mechanism of clearance involving C3b‐mediated opsonization of Aβ and binding by CD11b on microglia (1). This could be exacerbated if the variant in the CR1 gene negatively effects C3b homeostasis, which could result in the inability to inactivate C3b, leading to its accumulation. Upon delivery of the complex to cerebral blood vessels for clearance (2), Aβ may become deposited in the vessel wall caused by lack of proper phagocytosis (3) and C3b is allowed to propagate activation to the MAC (4), which would be even more pronounced with decreased defense mechanisms such as CD59 (5). The ultimate result is deposition of amyloid on the blood vessel wall and complement‐mediated cell lysis, both leading to hallmarks of this vasculopathy, CAA and microbleeds, respectively.
