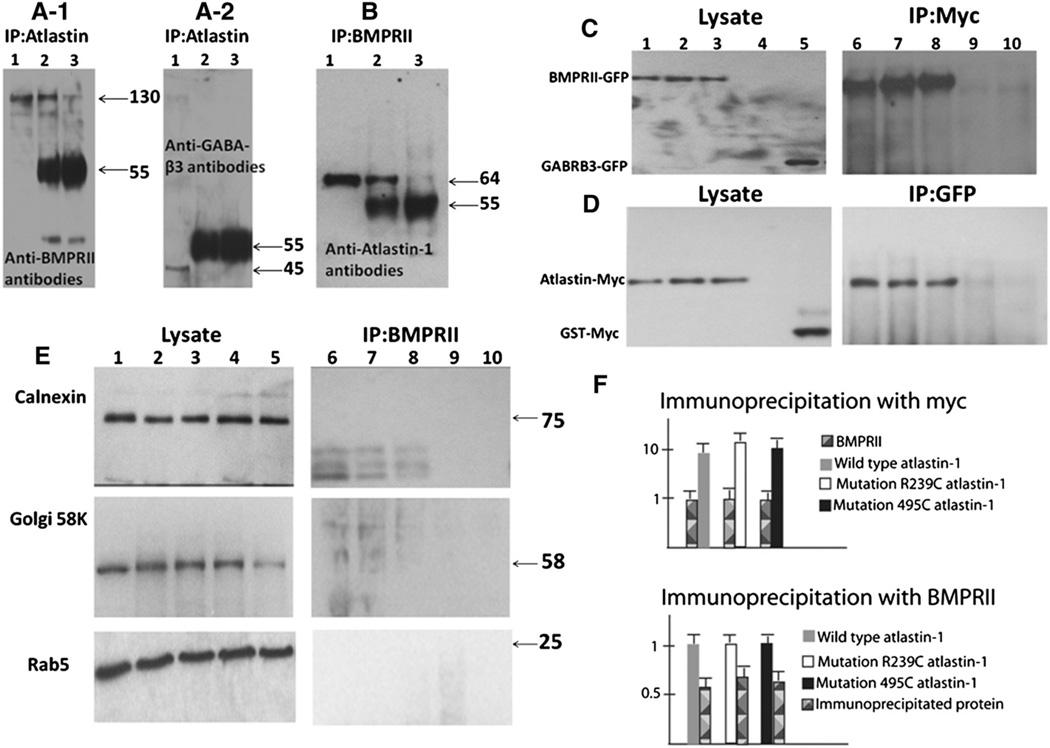
Fig. 1.
Atlastin-1 and BMPRII are binding partners. The whole rat brain extracts proteins were immunoprecipitated with atlastin-1 antibodies and analyzed by immunoblotting with BMPRII antibodies (panels A1), control GABAA-β3 antibodies (GABRB3) (panel A2), or immunoprecipitated with BMPRII antibodies and immunoblotted with atlastin-1 antibodies (panel B). Lane 1 shows studied proteins from cell lysates used for immunoprecipitation experiments, lane 2 antibodies used as the “bait” (atlastin-1 panels A-1 and A-2, BMPRII panel B) incubated with protein A/G agarose beads and lane 3 control experiments with protein A/G agarose beads alone. We observed 130 kDa band, corresponding to BMPRII (panel A-1, lane 2) and 64 kDa band, corresponding to atlastin-1 (panel B, lane 2), which were immunoprecipitated in the presence of bait antibodies. Non-specific background was observed mostly at the 55 kDa size. Very faint bands were also present in lane 3 in panels A-1 and B, again representing a non-specific background; however, lane 2 with co-immunoprecipitation had more than 100-fold higher activity in both instances. GABRB3 antibodies served as a control protein for the specificity of immunoprecipitation and we did not detect this protein after immunoprecipitation in lanes 2 and 3 (panel A-2). Panels C and D show analysis of HEK239-T cells cotransfected with BMPRII-GFP and WT atlastin-1-myc (lane 1), R239R atlastin-1:myc (lane 2), R459W atlastin-1:myc (lane 3). Control experiments included untransfected control cell lines (lane 4) and cotransfection of WT atlastin-myc with GABRB3-GFP (panel C, lane 5) or BMPRII-GFP and GST-myc (panel D, lane 5). Immunoprecipitation was done using anti-myc antibodies (panel C) or anti-GFP antibodies (panel D). Co-immunoprecipitation was only observed for coexpressed BMPRII-GFP and WT and mutant forms of atlastin-1:myc (panels C and D, lanes 6–8), while control proteins GABRB3 or GST did not interact with atlastin-1 or BMPRII (lanes 9 and 10). Blots from panels C and D were also analyzed with the markers of subcellular organelles where atlastin-1 can colocalize in order to detect membrane segments containing both BMPRII and atlastin-1 (panel E). We used markers for endoplasmatic reticulum (calnexin, upper row), Golgi complex (Golgi 58K, middle row) and early endosomes (Rab5, lower row). No signal was observed for BMPRII protein (right column) while cellular lysates showed a robust signal of used subcellular markers in all five lanes (left column). Introduced atlastin-1 mutations did not modify the interaction with BMPRII. Semiquantitative analysis after normalization of BMPRII signal from cell lysates (upper part of panel E) and after signal normalization from all three studied forms of atlastin-1 (lower part of panel E) did not reveal any statistically significant differences of signal intensity from immunoprecipitated partner proteins.