Figure 1.
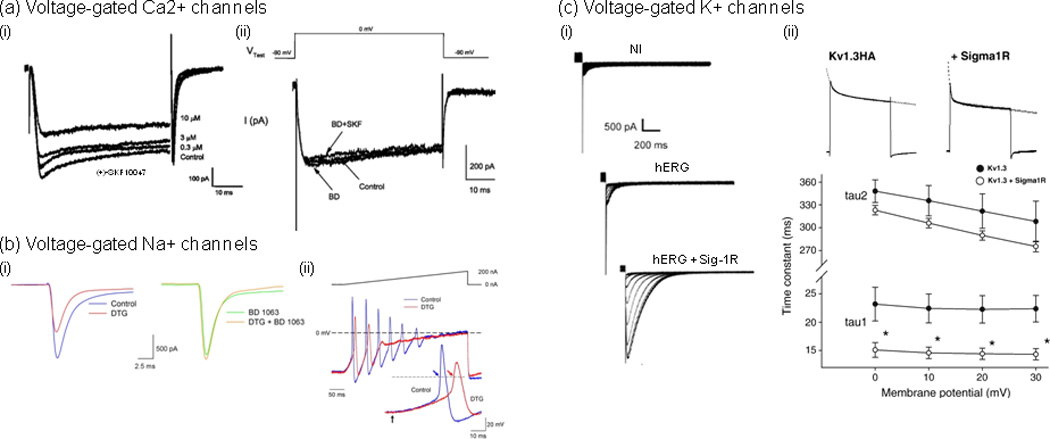
Examples of direct modulation of Ca2+, Na+ and K+ voltage-gated channels by Sig1Rs. (a) (i) The Sig1R agonist, (+)-SKF10047, inhibited voltage-gated Ca2+ currents in a concentration-dependent manner [18]. Whole-cell Ca2+ currents were recorded using patch clamp on cultured retinal ganglion cells (2 DIV) prepared from adult rats. Currents were evoked with a depolarization voltage step from −90 to 0 mV for 55 ms. (ii) (+)-SKF10047 inhibitory effect was prevented by the Sig-1R antagonist BD 1047. Treatment with BD 1047 alone had little effect on whole-cell Ca2+ currents. (b) (i), In rat intracardiac ganglion neurons, the Sig-1R antagonist BD 1063 blocked the effects of Sig-Rs pan-selective agonist 1,3-di(2-tolyl)guanidine (DTG) on peak Na+ currents [26]. Na+ currents were evoked by voltage steps from −90 to −10 mV in a single neuron in the absence (Control; left traces) and presence of 30 µM DTG (DTG; left traces), in the presence of 100 nM BD 1063 alone (BD 1063; right traces) and following co-application of both drugs (DTG+BD 1063; right traces). (ii) DTG-induced inhibition of Na+ currents resulted in delayed action potential latency and decreased firing rate. Action potentials generated by 400 ms depolarizing current ramps (0 to 200 nA) from a neuron in the absence (blue line) and presence of DTG (30 µM) (red line). Inset shows first action potentials generated by the ramps on an expanded time scale. Arrows in inset indicate start point of the injected current ramp and points at which latency times were measured. Dashed lines represent 0 mV, and the solid line above voltage traces represents the current ramp protocol used. (c) Sig-1Rs bidirectionally modulate K+ currents, which occurs through direct protein-protein interaction and results in either the modulation of K+ channels function [31, 33] or the regulation of subunits trafficking [32]. (i) Sig1R expression stimulates human ether-à-gogo K+ currents (hERG) in Xenopus oocytes [32]. Traces represent families of tail currents recorded in noninjected (NI), hERG cRNA-injected (hERG; 25 pg/oocyte), and hERG + Sig1R cRNA-injected (hERG + Sig1R; 25 pg and 5 ng/oocyte, respectively) oocytes (representative experiment). Tail currents were recorded following prepulses from −70 to 40 mV. (ii) Sig-1R co-expression accelerates Kv1.3 inactivation kinetics [33]. (i) Current decay of Kv1.3 alone and co-expressed with the Sig-1R were fitted with a double exponential function (dashed lines). (ii) A double exponential fit to the current decay showed a voltage-independent fast (A1*exp(−t/tau1)) and a voltage-dependent slow components (A2*exp(−t/tau2)) of inactivation time constants. Both time constants were smaller in the co-injected oocytes (open circles) than in those injected with Kv1.3 cRNA only (closed circles). The fast component demonstrated significant difference at all voltages (*p<0.05). Adapted, with permission, from [18] (a), [26] (b), [32] (c) (i) and [33] (c) (ii).
