Fig.1.
Different regulation of Vps34 complexes by glucose starvation in a manner dependent on AMPK.
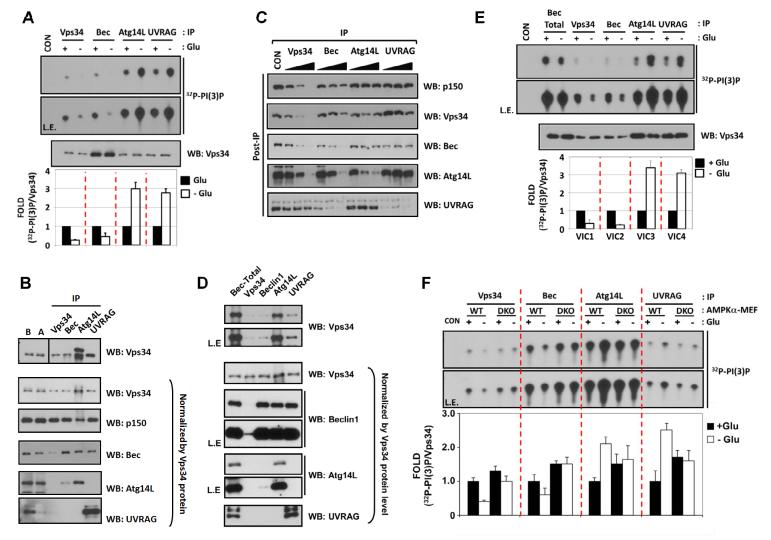
(A) Regulation of Vps34 complexes by glucose starvation. Wild-type MEF cells were starved for glucose (3 hrs) as indicated and the four Vps34-IP complexes were assayed for PI(3)P lipid kinase activity (n=4).
(B) Subunit composition of Vps34 complexes. Vps34 protein level in each preparation (Fig.1A) was firstly examined (Top) and then normalized to determine subunit composition of each complexes (lower panels). IP efficiency of each antibody was examined by comparison of the target protein amount in the lysate before (B) and after (A) IP.
(C) Relative abundance of Vps34 complexes. A quantitative immuno-depletion assay was performed with increasing amount of the indicated antibodies. Supernatants of MEF lysates after immuno-depletion (Post-IP) were examined to determine the level of Vps34 complex proteins.
(D) Preparation of four different highly-enriched Vps34 complexes. Subunit composition was examined as described in Fig.1B.
(E) Glucose starvation inhibits VIC1 and VIC2 but activates VIC3 and VIC4. Four different VPS34 complexes in Fig.1D were subjected to Vps34 lipid kinase assay (n=3).
(F) AMPK is required for Vps34 regulation by glucose starvation. Endogenous Vps34 complexes were immuno-purified from glucose-starved MEFs as Fig.1A. The Vps34 kinase activity was normalized by the Vps34 protein levels (Fig.S1D) (n=4). Data are represented as mean ± S.D.; CON, mouse IgG-IP as a negative control. See also Figure S1.